BACTIMICINA CHILDRENS ALLERGY
BACTIMICINA CHILDREN'S ALLERGY
eaf554d4-a758-4ed6-bec5-d6cbfedae408
HUMAN OTC DRUG LABEL
May 15, 2025
DLC Laboratories, Inc.
DUNS: 093351930
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DIPHENHYDRAMINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
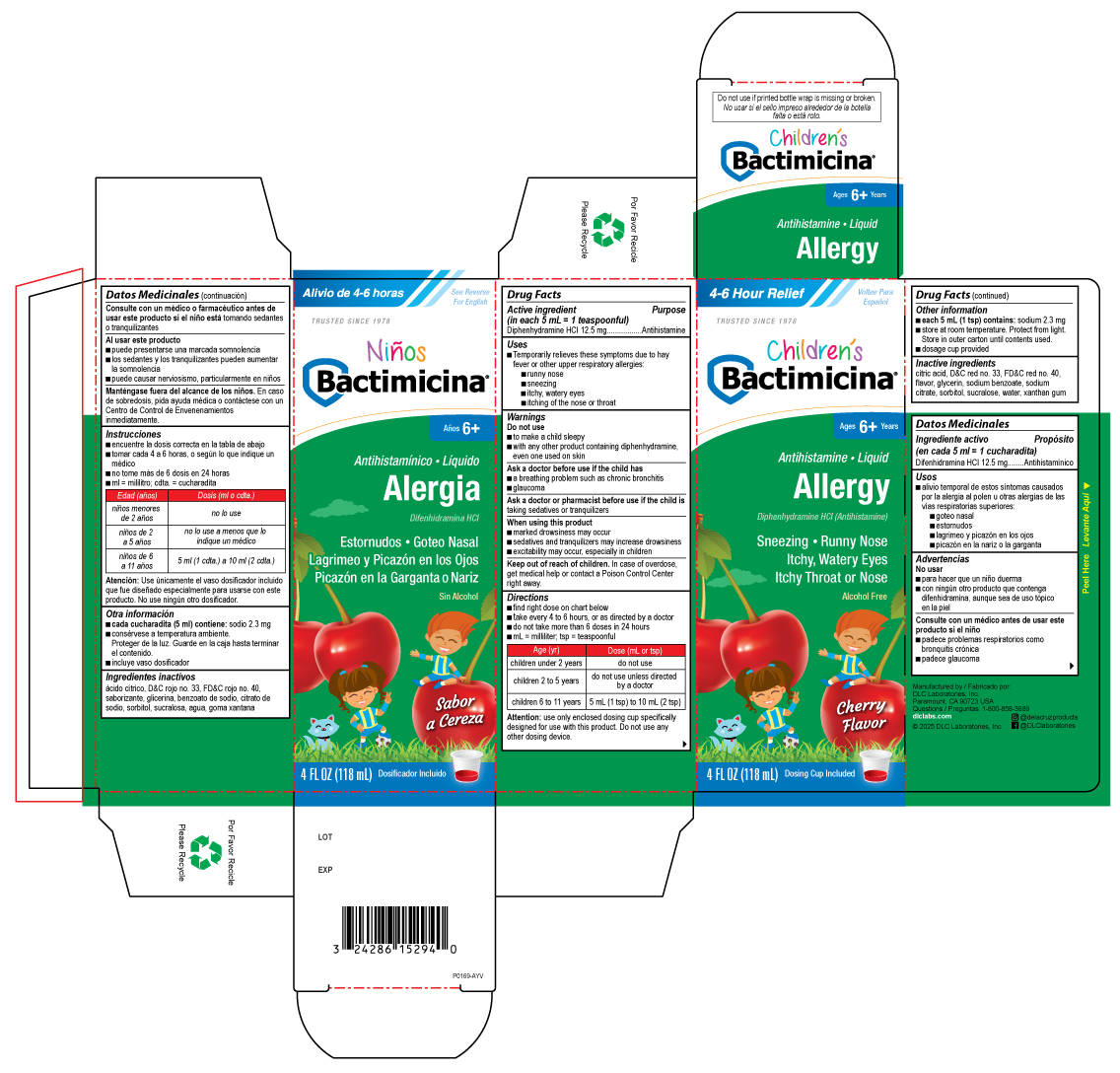
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 118 mL Bottle Carton
4-6 Hour Relief
Voltee Para
Español
TRUSTED SINCE 1978
Children's
Bactimicina®
Ages 6+ Years
Antihistamine • Liquid
Allergy
Diphenhydramine HCL (Antihistamine)
Sneezing • Runny Nose
Itchy, Watery Eyes
Itchy Throat or Nose
Alcohol Free
Cherry Flavor
4 FL OZ (118 mL) Dosing Cup Included
INDICATIONS & USAGE SECTION
Uses
- Temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
SPL UNCLASSIFIED SECTION
Manufactured by
DLC Laboratories, Inc.
Paramount, CA 90723 USA
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each 5 mL = 1 teaspoonful)
Diphenhydramine HCl 12.5 mg
OTC - PURPOSE SECTION
Purpose
Antihistamine
WARNINGS SECTION
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if the child has
- a breathing problem such as chronic bronchitis
- glaucoma
Ask a doctor or pharmacist before use if the child is taking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- sedatives and tranquilizers may increase drowsiness
- excitability may occur, especially in children
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- find right dose on chart below
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 doses in 24 hours
- mL = milliliter; tsp = teaspoonful
|
Age (yr) |
Dose (mL or tsp) |
|---|---|
|
children under 2 years |
do not use |
|
children 2 to 5 years |
do not use unless directed by a doctor |
|
children 6 to 11 years |
5 mL (1 tsp) to 10 mL (2 tsp) |
Attention: use only enclosed dosing cup specifically designed for use with this product. Do not use any other dosing device.
STORAGE AND HANDLING SECTION
Other information
*each 5 mL (1 tsp) contains: sodium 2.3 mg
- store at room temperature. Protect from light. Store in outer carton until contents used.
- dosage cup provided *do not use if printed bottle wrap is missing or broken
INACTIVE INGREDIENT SECTION
Inactive ingredients
citric acid, D&C red no. 33, FD&C red no. 40, flavor, glycerin, sodium benzoate, sodium citrate, sorbitol, sucralose, water, xanthan gum
OTC - QUESTIONS SECTION
Questions
1-800-858-3889
