Somnitabs
HTE - 1019 - 2025-0520
0ce0cb28-5ea1-43e5-8da1-02128b9989a5
HUMAN OTC DRUG LABEL
May 20, 2025
HARRIS TEETER
DUNS: 047279351
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DIPHENHYDRAMINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
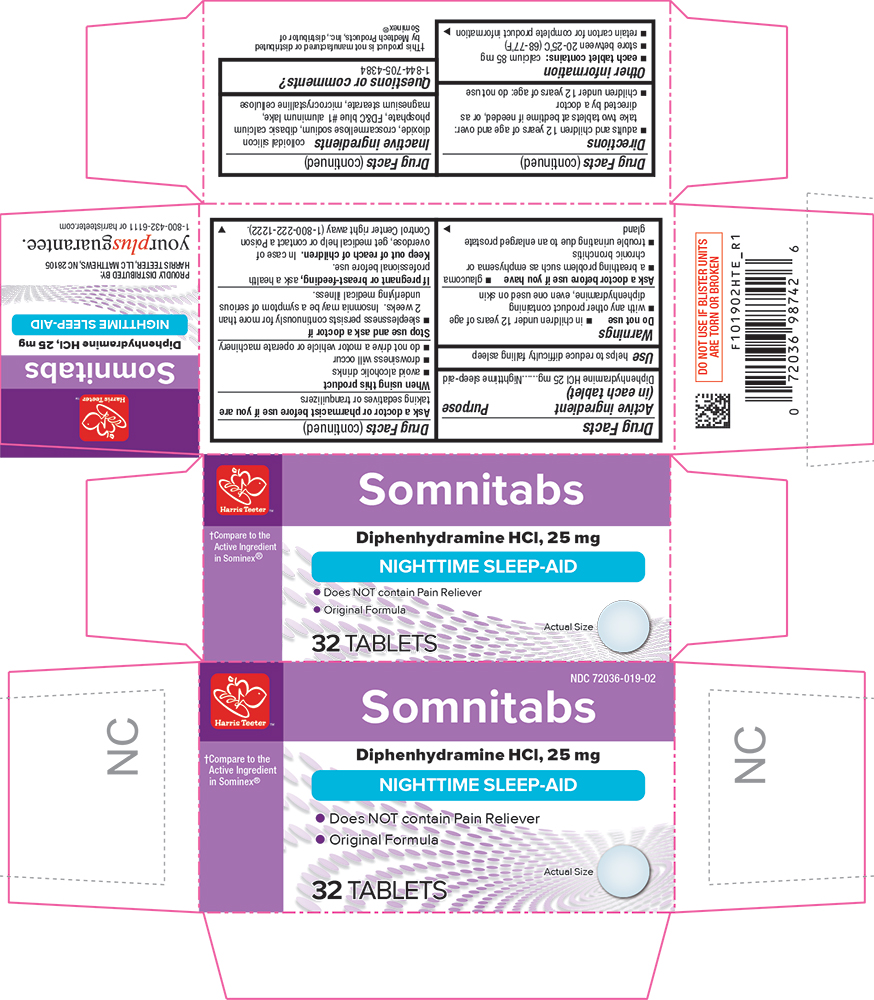
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Harris Teeter™
Somnitabs
Compare to the Active Ingredient in Sominex®
Diphenhydramine HCl, 25 mg
NIGHTTIME SLEEP-AID
•Does NOT contain Pain Reliever
• Original Formula
32 TABLETS
INDICATIONS & USAGE SECTION
Use
helps to reduce difficulty falling asleep
SPL UNCLASSIFIED SECTION
Drug Facts
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each tablet)
Diphenhydramine HCl 25 mg
OTC - PURPOSE SECTION
Purpose
Nighttime sleep-aid
WARNINGS SECTION
Warnings
Do not use
- in children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- trouble urinating due to an enlarged prostate gland
Ask a doctor or pharmacist before use if you aretaking sedatives or tranquilizers
When using this productavoid alcoholic drinks
Stop use and ask a doctor ifsleeplessness persists continuously for more than 2 weeks. Insomnia may be a symptom of serious underlying medical illness.
**If pregnant or breast-feeding,**ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 12 years of age and over: take 2 tablets at bedtime if needed, or as directed by a doctor
STORAGE AND HANDLING SECTION
Other information
- each tablet contains:calcium 85 mg
- store at room temperature 15°-30°C (59°-86°F)
- retain carton for complete product information
INACTIVE INGREDIENT SECTION
Inactive ingredients
colloidal silicon dioxide, croscarmellose sodium, dibasic calcium phosphate, FD&C blue #1, magnesium stearate, microcrystalline cellulose
OTC - QUESTIONS SECTION
Questions or comments?
1-844-705-4384
