INFLIXIMAB
These highlights do not include all the information needed to use INFLIXIMAB safely and effectively. See full prescribing information for INFLIXIMAB. INFLIXIMAB for injection, for intravenous use Initial U.S. Approval: 1998
b05c174f-832c-4321-b34f-2c4ad3742269
HUMAN PRESCRIPTION DRUG LABEL
Oct 15, 2021
Janssen Biotech, Inc.
DUNS: 099091753
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
INFLIXIMAB
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
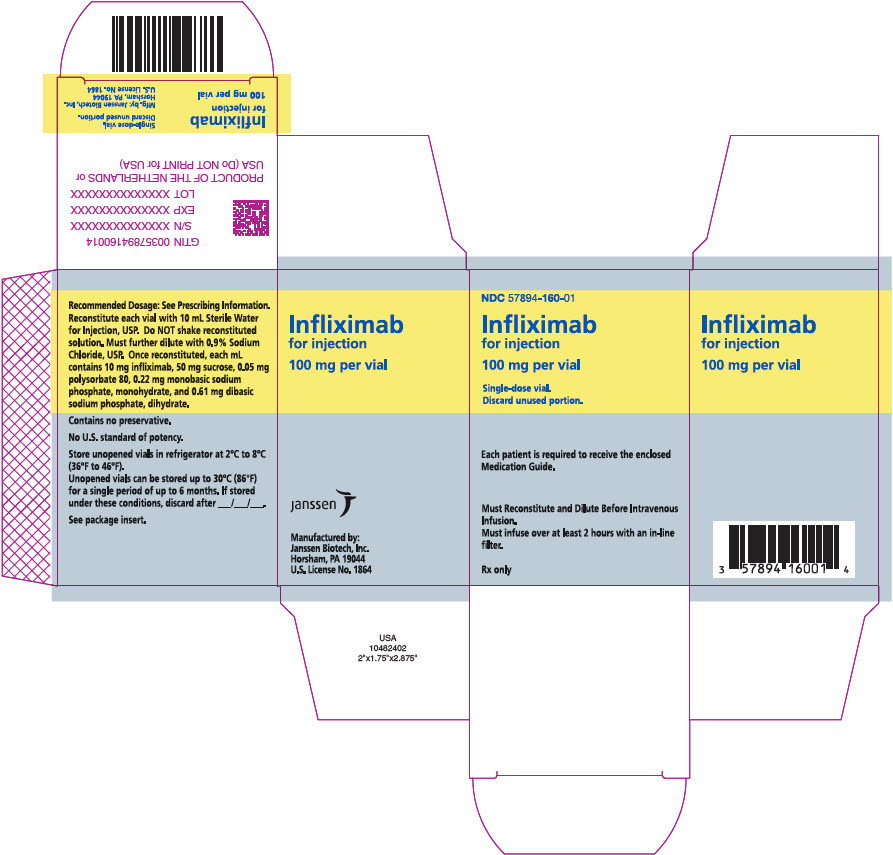
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Vial Box
NDC 57894-160-01
Infliximab
for injection
100 mg per vial
Single-dose vial.
Discard unused portion.
Each patient is required to receive the enclosed
Medication Guide.
Must Reconstitute and Dilute Before Intravenous
Infusion.
Must infuse over at least 2 hours with an in-line
filter.
Rx only
BOXED WARNING SECTION
WARNING: SERIOUS INFECTIONS and MALIGNANCY
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
The use of Infliximab at doses >5 mg/kg is contraindicated in patients with moderate or severe heart failure [see Warnings and Precautions (5.5) and Adverse Reactions (6.1)].
Infliximab is contraindicated in patients with a previous severe hypersensitivity reaction to infliximab or any of the inactive ingredients of Infliximab or any murine proteins [severe hypersensitivity reactions have included anaphylaxis, hypotension, and serum sickness] [see Warnings and Precautions (5.7) and Adverse Reactions (6.1)].
- Infliximab doses >5 mg/kg in moderate or severe heart failure. (4)
- Previous severe hypersensitivity reaction to infliximab or any inactive ingredients of Infliximab or to any murine proteins. (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adults
The data described herein reflect exposure to Infliximab in 4779 adult patients (1304 patients with RA, 1106 patients with CD, 202 with AS, 293 with PsA, 484 with UC, 1373 with Ps, and 17 patients with other conditions), including 2625 patients exposed beyond 30 weeks and 374 exposed beyond 1 year. [For information on adverse reactions in pediatric patients see Adverse Reactions (6.1)]. One of the most-common reasons for discontinuation of treatment was infusion-related reactions (e.g., dyspnea, flushing, headache and rash).
Infusion-Related Reactions
Adverse Reactions During or Shortly After Infusion
An infusion reaction was defined in clinical trials as any adverse event occurring during an infusion or within 1 hour after an infusion. In all the clinical studies, approximately 20% of Infliximab-treated patients experienced an infusion reaction compared with 10% of placebo-treated patients. Of Infliximab-treated patients who had an infusion reaction during the induction period, 27% experienced an infusion reaction during the maintenance period. Of patients who did not have an infusion reaction during the induction period, 9% experienced an infusion reaction during the maintenance period.
Among all Infliximab infusions, 3% were accompanied by nonspecific symptoms such as fever or chills, 1% were accompanied by cardiopulmonary reactions (primarily chest pain, hypotension, hypertension or dyspnea), and <1% were accompanied by pruritus, urticaria, or the combined symptoms of pruritus/urticaria and cardiopulmonary reactions. Serious infusion reactions occurred in <1% of patients and included anaphylaxis, convulsions, erythematous rash and hypotension. Approximately 3% of patients discontinued Infliximab because of infusion reactions, and all patients recovered with treatment and/or discontinuation of the infusion. Infliximab infusions beyond the initial infusion were not associated with a higher incidence of reactions. The infusion reaction rates remained stable in Ps through 1 year in Ps Study I. In psoriasis Study II, the rates were variable over time and somewhat higher following the final infusion than after the initial infusion. Across the 3 Ps studies, the percent of total infusions resulting in infusion reactions (i.e., an adverse event occurring within 1 hour) was 7% in the 3 mg/kg group, 4% in the 5 mg/kg group, and 1% in the placebo group.
Patients who became positive for antibodies to infliximab were more likely (approximately two-to three-fold) to have an infusion reaction than were those who were negative. Use of concomitant immunosuppressant agents appeared to reduce the frequency of both antibodies to infliximab and infusion reactions [see Adverse Reactions (6.2) and Drug Interactions (7.3)].
Infusion Reactions Following Re-administration
In a clinical trial of patients with moderate to severe Ps designed to assess the efficacy of long-term maintenance therapy versus re-treatment with an induction regimen of Infliximab following disease flare, 4% (8/219) of patients in the re-treatment induction therapy arm experienced serious infusion reactions versus <1% (1/222) in the maintenance therapy arm. Patients enrolled in this trial did not receive any concomitant immunosuppressant therapy. In this study, the majority of serious infusion reactions occurred during the second infusion at Week 2. Symptoms included, but were not limited to, dyspnea, urticaria, facial edema, and hypotension. In all cases, Infliximab treatment was discontinued and/or other treatment instituted with complete resolution of signs and symptoms.
Delayed Reactions/Reactions Following Re-administration
In Ps studies, approximately 1% of Infliximab-treated patients experienced a possible delayed hypersensitivity reaction, generally reported as serum sickness or a combination of arthralgia and/or myalgia with fever and/or rash. These reactions generally occurred within 2 weeks after repeat infusion.
Infections
In Infliximab clinical studies, treated infections were reported in 36% of Infliximab-treated patients (average of 51 weeks of follow-up) and in 25% of placebo-treated patients (average of 37 weeks of follow-up). The infections most frequently reported were respiratory tract infections (including sinusitis, pharyngitis, and bronchitis) and urinary tract infections. Among Infliximab-treated patients, serious infections included pneumonia, cellulitis, abscess, skin ulceration, sepsis, and bacterial infection. In clinical trials, 7 opportunistic infections were reported; 2 cases each of coccidioidomycosis (1 case was fatal) and histoplasmosis (1 case was fatal), and 1 case each of pneumocystosis, nocardiosis and cytomegalovirus. Tuberculosis (TB) was reported in 14 patients, 4 of whom died due to miliary tuberculosis. Other cases of TB, including disseminated TB, also have been reported post-marketing. Most of these cases of TB occurred within the first 2 months after initiation of therapy with Infliximab and may reflect recrudescence of latent disease [see Warnings and Precautions (5.1)]. In the 1-year placebo-controlled studies RA I and RA II, 5.3% of patients receiving Infliximab every 8 weeks with MTX developed serious infections as compared to 3.4% of placebo patients receiving MTX. Of 924 patients receiving Infliximab, 1.7% developed pneumonia and 0.4% developed TB, when compared to 0.3% and 0.0% in the placebo arm respectively. In a shorter (22-week) placebo-controlled study of 1082 RA patients randomized to receive placebo, 3 mg/kg or 10 mg/kg Infliximab infusions at 0, 2, and 6 weeks, followed by every 8 weeks with MTX, serious infections were more frequent in the 10 mg/kg Infliximab group (5.3%) than the 3 mg/kg or placebo groups (1.7% in both). During the 54-week Crohn's II Study, 15% of patients with fistulizing CD developed a new fistula-related abscess.
In Infliximab clinical studies in patients with UC, infections treated with antimicrobials were reported in 27% of Infliximab-treated patients (average of 41 weeks of follow-up) and in 18% of placebo-treated patients (average 32 weeks of follow-up). The types of infections, including serious infections, reported in patients with UC were similar to those reported in other clinical studies.
The onset of serious infections may be preceded by constitutional symptoms such as fever, chills, weight loss, and fatigue. The majority of serious infections, however, may also be preceded by signs or symptoms localized to the site of the infection.
Autoantibodies/Lupus-like Syndrome
Approximately half of Infliximab-treated patients in clinical trials who were antinuclear antibody (ANA) negative at baseline developed a positive ANA during the trial compared with approximately one-fifth of placebo-treated patients. Anti-dsDNA antibodies were newly detected in approximately one-fifth of Infliximab-treated patients compared with 0% of placebo-treated patients. Reports of lupus and lupus-like syndromes, however, remain uncommon.
Malignancies
In controlled trials, more Infliximab-treated patients developed malignancies than placebo-treated patients [see Warnings and Precautions (5.2)].
In a randomized controlled clinical trial exploring the use of Infliximab in patients with moderate to severe COPD who were either current smokers or ex- smokers, 157 patients were treated with Infliximab at doses similar to those used in RA and CD. Of these Infliximab-treated patients, 9 developed a malignancy, including 1 lymphoma, for a rate of 7.67 cases per 100 patient- years of follow-up (median duration of follow-up 0.8 years; 95% CI 3.51 – 14.56). There was 1 reported malignancy among 77 control patients for a rate of 1.63 cases per 100 patient-years of follow-up (median duration of follow-up 0.8 years; 95% CI 0.04 – 9.10). The majority of the malignancies developed in the lung or head and neck [see Warnings and Precautions (5.2)].
Adverse Reactions in Patients with NYHA Class III/IV Heart Failure
In a randomized, double-blind study evaluating Infliximab in moderate or severe heart failure (NYHA Class III/IV; left ventricular ejection fraction ≤35%), 150 patients were randomized to receive treatment with 3 infusions of Infliximab 10 mg/kg, 5 mg/kg, or placebo, at 0, 2, and 6 weeks. Higher incidences of mortality and hospitalization due to worsening heart failure were observed in patients receiving the 10 mg/kg Infliximab dose. At 1 year, 8 patients in the 10 mg/kg Infliximab group had died compared with 4 deaths each in the 5 mg/kg Infliximab and the placebo groups. There were trends toward increased dyspnea, hypotension, angina, and dizziness in both the 10 mg/kg and 5 mg/kg Infliximab treatment groups, versus placebo. Infliximab has not been studied in patients with mild heart failure (NYHA Class I/II) [see Contraindications (4) and Warnings and Precautions (5.5)].
Hepatotoxicity
Severe liver injury, including acute liver failure and autoimmune hepatitis, has been reported in patients receiving Infliximab [see Warnings and Precautions (5.4)]. Reactivation of hepatitis B virus has occurred in patients receiving TNF blockers, including Infliximab, who are chronic carriers of this virus [see Warnings and Precautions (5.3)].
In clinical trials in RA, CD, UC, AS, Ps, and PsA, elevations of aminotransferases were observed (ALT more common than AST) in a greater proportion of patients receiving Infliximab than in controls (Table 1), both when Infliximab was given as monotherapy and when it was used in combination with other immunosuppressive agents. In general, patients who developed ALT and AST elevations were asymptomatic, and the abnormalities decreased or resolved with either continuation or discontinuation of Infliximab, or modification of concomitant medications.
Table 1: Proportion of Patients with Elevated ALT in Clinical Trials in Adults|
Proportion of patients with elevated ALT | ||||||
|---|---|---|---|---|---|---|
|
≥3 × ULN |
≥5 × ULN | ||||
|
Placebo |
Infliximab |
Placebo |
Infliximab |
Placebo |
Infliximab | |
| ||||||
|
Rheumatoid arthritis* |
24% |
34% |
3% |
4% |
<1% |
<1% |
|
Crohn's disease† |
34% |
39% |
4% |
5% |
0% |
2% |
|
Ulcerative colitis‡ |
12% |
17% |
1% |
2% |
<1% |
<1% |
|
Ankylosing spondylitis§ |
15% |
51% |
0% |
10% |
0% |
4% |
|
Psoriatic arthritis¶ |
16% |
50% |
0% |
7% |
0% |
2% |
|
Plaque psoriasis# |
24% |
49% |
<1% |
8% |
0% |
3% |
Adverse Reactions in Psoriasis Studies
During the placebo-controlled portion across the 3 clinical trials up to Week 16, the proportion of patients who experienced at least 1 serious adverse reaction (SAE; defined as resulting in death, life threatening, requires hospitalization, or persistent or significant disability/incapacity) was 0.5% in the 3 mg/kg Infliximab group, 1.9% in the placebo group, and 1.6% in the 5 mg/kg Infliximab group.
Among patients in the 2 Phase 3 studies, 12.4% of patients receiving Infliximab 5 mg/kg every 8 weeks through 1 year of maintenance treatment experienced at least 1 SAE in Study I. In Study II, 4.1% and 4.7% of patients receiving Infliximab 3 mg/kg and 5 mg/kg every 8 weeks, respectively, through 1 year of maintenance treatment experienced at least 1 SAE.
One death due to bacterial sepsis occurred 25 days after the second infusion of 5 mg/kg Infliximab. Serious infections included sepsis, and abscesses. In Study I, 2.7% of patients receiving Infliximab 5 mg/kg every 8 weeks through 1 year of maintenance treatment experienced at least 1 serious infection. In Study II, 1.0% and 1.3% of patients receiving Infliximab 3 mg/kg and 5 mg/kg, respectively, through 1 year of treatment experienced at least 1 serious infection. The most common serious infection (requiring hospitalization) was abscess (skin, throat, and peri-rectal) reported by 5 (0.7%) patients in the 5 mg/kg Infliximab group. Two active cases of tuberculosis were reported: 6 weeks and 34 weeks after starting Infliximab.
In the placebo-controlled portion of the Ps studies, 7 of 1123 patients who received Infliximab at any dose were diagnosed with at least one NMSC compared to 0 of 334 patients who received placebo.
In the Ps studies, 1% (15/1373) of patients experienced serum sickness or a combination of arthralgia and/or myalgia with fever, and/or rash, usually early in the treatment course. Of these patients, 6 required hospitalization due to fever, severe myalgia, arthralgia, swollen joints, and immobility.
Other Adverse Reactions in Adults
Safety data are available from 4779 Infliximab-treated adult patients, including 1304 with RA, 1106 with CD, 484 with UC, 202 with AS, 293 with PsA, 1373 with Ps and 17 with other conditions. [For information on other adverse reactions in pediatric patients, see Adverse Reactions (6.1)]. Adverse reactions reported in ≥5% of all patients with RA receiving 4 or more infusions are in Table 2. The types and frequencies of adverse reactions observed were similar in Infliximab-treated RA, AS, PsA, Ps, and CD patients except for abdominal pain, which occurred in 26% of Infliximab-treated patients with CD. In the CD studies, there were insufficient numbers and duration of follow-up for patients who never received Infliximab to provide meaningful comparisons.
Table 2: Adverse Reactions that Occurred in ≥ 5% of Patients who Received ≥ 4 Infliximab Infusions for RA|
Placebo |
Infliximab | |
|---|---|---|
|
(n=350) |
(n=1129) | |
|
Average weeks of follow-up |
59 weeks |
66 weeks |
|
Upper respiratory tract infection |
25% |
32% |
|
Nausea |
20% |
21% |
|
Headache |
14% |
18% |
|
Sinusitis |
8% |
14% |
|
Diarrhea |
12% |
12% |
|
Abdominal pain |
8% |
12% |
|
Pharyngitis |
8% |
12% |
|
Coughing |
8% |
12% |
|
Bronchitis |
9% |
10% |
|
Rash |
5% |
10% |
|
Dyspepsia |
7% |
10% |
|
Fatigue |
7% |
9% |
|
Urinary tract infection |
6% |
8% |
|
Pain |
7% |
8% |
|
Arthralgia |
7% |
8% |
|
Pruritus |
2% |
7% |
|
Fever |
4% |
7% |
|
Hypertension |
5% |
7% |
|
Moniliasis |
3% |
5% |
The most common serious adverse reactions observed in clinical trials were infections [see Adverse Reactions (6.1)]. Other serious, medically relevant adverse reactions ≥0.2% or clinically significant adverse reactions by body system were as follows:
- Body as a whole: allergic reaction, edema
- Blood: pancytopenia
- Cardiovascular: hypotension
- Gastrointestinal: constipation, intestinal obstruction
- Central and Peripheral Nervous: dizziness
- Heart Rate and Rhythm: bradycardia
- Liver and Biliary: hepatitis
- Metabolic and Nutritional: dehydration
- Platelet, Bleeding and Clotting: thrombocytopenia
- Neoplasms: lymphoma
- Red Blood Cell: anemia, hemolytic anemia
- Resistance Mechanism: cellulitis, sepsis, serum sickness, sarcoidosis
- Respiratory: lower respiratory tract infection (including pneumonia), pleurisy, pulmonary edema
- Skin and Appendages: increased sweating
- Vascular (Extracardiac): thrombophlebitis
- White Cell and Reticuloendothelial: leukopenia, lymphadenopathy
Adverse Reactions in Pediatric Patients
Adverse Reactions in Pediatric Patients with Crohn's Disease
There were some differences in the adverse reactions observed in the pediatric patients receiving Infliximab compared to those observed in adults with CD. These differences are discussed in the following paragraphs.
The following adverse reactions were reported more commonly in 103 randomized pediatric CD patients administered 5 mg/kg Infliximab through 54 weeks than in 385 adult CD patients receiving a similar treatment regimen: anemia (11%), leukopenia (9%), flushing (9%), viral infection (8%), neutropenia (7%), bone fracture (7%), bacterial infection (6%), and respiratory tract allergic reaction (6%).
Infections were reported in 56% of randomized pediatric patients in Study Peds Crohn's and in 50% of adult patients in Study Crohn's I. In Study Peds Crohn's, infections were reported more frequently for patients who received every 8-week as opposed to every 12-week infusions (74% and 38%, respectively), while serious infections were reported for 3 patients in the every 8-week and 4 patients in the every 12-week maintenance treatment group. The most commonly reported infections were upper respiratory tract infection and pharyngitis, and the most commonly reported serious infection was abscess. Pneumonia was reported for 3 patients, (2 in the every 8-week and 1 in the every 12-week maintenance treatment groups). Herpes zoster was reported for 2 patients in the every 8-week maintenance treatment group.
In Study Peds Crohn's, 18% of randomized patients experienced 1 or more infusion reactions, with no notable difference between treatment groups. Of the 112 patients in Study Peds Crohn's, there were no serious infusion reactions, and 2 patients had non-serious anaphylactoid reactions.
Elevations of ALT up to 3 times the upper limit of normal (ULN) were seen in 18% of pediatric patients in CD clinical trials; 4% had ALT elevations ≥3 × ULN, and 1% had elevations ≥5 × ULN. (Median follow-up was 53 weeks).
Adverse Reactions in Pediatric Patients with Ulcerative Colitis
Overall, the adverse reactions reported in the pediatric UC trial and adult UC (Study UC I and Study UC II) studies were generally consistent. In a pediatric UC trial, the most common adverse reactions were upper respiratory tract infection, pharyngitis, abdominal pain, fever, and headache.
Infections were reported in 31 (52%) of 60 treated patients in the pediatric UC trial and 22 (37%) required oral or parenteral antimicrobial treatment. The proportion of patients with infections in the pediatric UC trial was similar to that in the pediatric CD study (Study Peds Crohn's) but higher than the proportion in the adults' UC studies (Study UC I and Study UC II). The overall incidence of infections in the pediatric UC trial was 13/22 (59%) in the every 8 week maintenance treatment group. Upper respiratory tract infection (7/60 [12%]) and pharyngitis (5/60 [8%]) were the most frequently reported respiratory system infections. Serious infections were reported in 12% (7/60) of all treated patients.
Elevations of ALT up to 3 times the upper limit of normal (ULN) were seen in 17% (10/60) of pediatric patients in the pediatric UC trial; 7% (4/60) had ALT elevations ≥3 × ULN, and 2% (1/60) had elevations ≥5 × ULN (median follow-up was 49 weeks).
Overall, 8 of 60 (13%) treated patients experienced one or more infusion reactions, including 4 of 22 (18%) patients in the every 8-week treatment maintenance group. No serious infusion reactions were reported.
In the pediatric UC trial, 45 patients were in the 12 to 17 year age group and 15 in the 6 to 11 year age group. The numbers of patients in each subgroup are too small to make any definitive conclusions about the effect of age on safety events. There were higher proportions of patients with serious adverse events (40% vs. 18%) and discontinuation due to adverse events (40% vs. 16%) in the younger age group than in the older age group. While the proportion of patients with infections was also higher in the younger age group (60% vs. 49%), for serious infections, the proportions were similar in the two age groups (13% in the 6 to 11 year age group vs. 11% in the 12 to 17 year age group). Overall proportions of adverse reactions, including infusion reactions, were similar between the 6 to 11 and 12 to 17 year age groups (13%).
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other infliximab products may be misleading.
Treatment with Infliximab can be associated with the development of antibodies to infliximab. An enzyme immunoassay (EIA) method was originally used to measure anti-infliximab antibodies in clinical studies of Infliximab. The EIA method is subject to interference by serum infliximab, possibly resulting in an underestimation of the rate of patient antibody formation. A separate, drug-tolerant electrochemiluminescence immunoassay (ECLIA) method for detecting antibodies to infliximab was subsequently developed and validated. This method is 60-fold more sensitive than the original EIA. With the ECLIA method, all clinical samples can be classified as either positive or negative for antibodies to infliximab without the need for the inconclusive category.
The incidence of antibodies to infliximab was based on the original EIA method in all clinical studies of Infliximab except for the Phase 3 study in pediatric patients with UC where the incidence of antibodies to infliximab was detected using both the EIA and ECLIA methods.
Immunogenicity in Adult Patients
The incidence of antibodies to infliximab in patients with RA and CD given a 3-dose induction regimen followed by maintenance dosing was approximately 10% as assessed through 1 to 2 years of Infliximab treatment. A higher incidence of antibodies to infliximab was observed in CD patients receiving Infliximab after drug-free intervals >16 weeks. In a PsA study in which 191 patients received 5 mg/kg with or without MTX, antibodies to infliximab occurred in 15% of patients. The majority of antibody-positive patients had low titers. Antibody development was lower among RA and CD patients receiving immunosuppressant therapies such as 6-MP/AZA or MTX. Patients who were antibody-positive were more likely to have higher rates of clearance, have reduced efficacy, and to experience an infusion reaction than were patients who were antibody negative [see Adverse Reactions (6.1)]. In the Ps Study II, which included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 36% of patients treated with 5 mg/kg every 8 weeks for 1 year, and in 51% of patients treated with 3 mg/kg every 8 weeks for 1 year.
In the Ps Study III, which also included both the 5 mg/kg and 3 mg/kg doses, antibodies were observed in 20% of patients treated with 5 mg/kg induction (weeks 0, 2 and 6), and in 27% of patients treated with 3 mg/kg induction. Despite the increase in antibody formation, the infusion reaction rates in Studies I and II in patients treated with 5 mg/kg induction followed by every 8 week maintenance for 1 year and in Study III in patients treated with 5 mg/kg induction (14.1%–23.0%) and serious infusion reaction rates (<1%) were similar to those observed in other study populations. The clinical significance of apparent increased immunogenicity on efficacy and infusion reactions in Ps patients as compared to patients with other diseases treated with Infliximab over the long term is not known.
Immunogenicity in Pediatric Patients with Crohn's Disease
In Study Peds Crohn's, in which all patients received stable doses of 6-MP, AZA, or MTX, excluding inconclusive samples, 3 of 24 patients had antibodies to infliximab. Although 105 patients were tested for antibodies to infliximab, 81 patients were classified as inconclusive because they could not be ruled as negative due to assay interference by the presence of infliximab in the sample.
Immunogenicity in Pediatric Patients with Ulcerative Colitis
In the pediatric UC trial, 58 patients were evaluated for antibodies to infliximab using the EIA as well as the drug-tolerant ECLIA. With the EIA, 4 of 58 (7%) patients had antibodies to infliximab. With the ECLIA, 30 of 58 (52%) patients had antibodies to infliximab. The higher incidence of antibodies to infliximab by the ECLIA method was due to the 60-fold higher sensitivity compared to the EIA method. While EIA-positive patients generally had undetectable trough infliximab concentrations, ECLIA-positive patients could have detectable trough concentrations of infliximab because the ECLIA assay is more sensitive and drug-tolerant.
6.3 Postmarketing Experience
Adverse reactions, some with fatal outcomes, have been identified during post approval use of Infliximab in adult and pediatric patients. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Postmarketing Adverse Reactions in Adults and Pediatric Patients
- Neutropenia [see Warnings and Precautions (5.6)], agranulocytosis (including infants exposed in utero to infliximab), idiopathic thrombocytopenic purpura, thrombotic thrombocytopenic purpura.
- Interstitial lung disease (including pulmonary fibrosis/interstitial pneumonitis and rapidly progressive disease).
- Pericardial effusion, systemic and cutaneous vasculitis.
- Erythema multiforme, Stevens-Johnson Syndrome, toxic epidermal necrolysis, linear IgA bullous dermatosis (LABD), acute generalized exanthematous pustulosis (AGEP), new onset and worsening psoriasis (all subtypes including pustular, primarily palmoplantar), lichenoid reactions.
- Peripheral demyelinating disorders (such as Guillain-Barré syndrome, chronic inflammatory demyelinating polyneuropathy, and multifocal motor neuropathy) transverse myelitis, and neuropathies (additional neurologic reactions have also been observed) [see Warnings and Precautions (5.9)].
- Acute liver failure, jaundice, hepatitis, and cholestasis [see Warnings and Precautions (5.4)].
- Serious infections [see Warnings and Precautions (5.1)] and vaccine breakthrough infection including bovine tuberculosis (disseminated BCG infection) following vaccination in an infant exposed in utero to infliximab [see Warnings and Precautions (5.13)].
- Malignancies, including leukemia, melanoma, Merkel cell carcinoma, and cervical cancer [see Warnings and Precautions (5.2)].
- Anaphylactic reactions, including anaphylactic shock, laryngeal/pharyngeal edema and severe bronchospasm, and seizure have been associated with Infliximab administration.
- Transient visual loss have been reported in association with Infliximab during or within 2 hours of infusion. Cerebrovascular accidents, myocardial ischemia/infarction (some fatal), and arrhythmia occurring within 24 hours of initiation of infusion have also been reported [see Warnings and Precautions (5.8)].
Postmarketing Serious Adverse Reactions in Pediatric Patients
The following serious adverse reactions have been reported in the post- marketing experience in pediatric patients: infections (some fatal) including opportunistic infections and tuberculosis, infusion reactions, hypersensitivity reactions, malignancies, including hepatosplenic T-cell lymphomas [see Boxed Warning and Warnings and Precautions (5.2)], transient hepatic enzyme abnormalities, lupus-like syndromes, and the development of autoantibodies.
Most common adverse reactions (>10%) – infections (e.g. upper respiratory, sinusitis, and pharyngitis), infusion-related reactions, headache, and abdominal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Biotech, Inc. at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088****or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Other Biological Products
The combination of Infliximab with other biological products used to treat the same conditions as Infliximab is not recommended [see Warnings and Precautions (5.10)].
An increased risk of serious infections was seen in clinical studies of other TNF blockers used in combination with anakinra or abatacept, with no added clinical benefit. Because of the nature of the adverse reactions seen with these combinations with TNF blocker therapy, similar toxicities may also result from the combination of anakinra or abatacept with other TNF blockers. Therefore, the combination of Infliximab and anakinra or abatacept is not recommended [see Warnings and Precautions (5.10)].
The concomitant use of tocilizumab with biological DMARDs such as TNF antagonists, including Infliximab, should be avoided because of the possibility of increased immunosuppression and increased risk of infection.
7.2 Methotrexate and Other Concomitant Medications
Specific drug interaction studies, including interactions with methotrexate (MTX), have not been conducted. The majority of patients in RA or CD clinical studies received one or more concomitant medications. In RA, concomitant medications besides MTX were nonsteroidal anti-inflammatory agents (NSAIDs), folic acid, corticosteroids and/or narcotics. Concomitant CD medications were antibiotics, antivirals, corticosteroids, 6-MP/AZA and aminosalicylates. In PsA clinical trials, concomitant medications included MTX in approximately half of the patients as well as NSAIDs, folic acid and corticosteroids. Concomitant MTX use may decrease the incidence of anti-infliximab antibody production and increase infliximab concentrations.
7.3 Immunosuppressants
Patients with CD who received immunosuppressants tended to experience fewer infusion reactions compared to patients on no immunosuppressants [see Adverse Reactions (6.1)]. Serum infliximab concentrations appeared to be unaffected by baseline use of medications for the treatment of CD including corticosteroids, antibiotics (metronidazole or ciprofloxacin) and aminosalicylates.
7.4 Cytochrome P450 Substrates
The formation of CYP450 enzymes may be suppressed by increased levels of cytokines (e.g., TNFα, IL-1, IL-6, IL-10, IFN) during chronic inflammation. Therefore, it is expected that for a molecule that antagonizes cytokine activity, such as infliximab, the formation of CYP450 enzymes could be normalized. Upon initiation or discontinuation of Infliximab in patients being treated with CYP450 substrates with a narrow therapeutic index, monitoring of the effect (e.g., warfarin) or drug concentration (e.g., cyclosporine or theophylline) is recommended and the individual dose of the drug product may be adjusted as needed.
7.5 Live Vaccines/Therapeutic Infectious Agents
It is recommended that live vaccines not be given concurrently with Infliximab. It is also recommended that live vaccines not be given to infants after in utero exposure to infliximab for at least 6 months following birth [see Warnings and Precautions (5.13)].
It is recommended that therapeutic infectious agents not be given concurrently with Infliximab [see Warnings and Precautions (5.13)].
Other Biological Products– increased risk of serious infections (7.1)
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Infliximab neutralizes the biological activity of TNFα by binding with high affinity to the soluble and transmembrane forms of TNFα and inhibits binding of TNFα with its receptors. Infliximab does not neutralize TNFβ (lymphotoxin-α), a related cytokine that utilizes the same receptors as TNFα. Biological activities attributed to TNFα include: induction of pro- inflammatory cytokines such as interleukins (IL) 1 and 6, enhancement of leukocyte migration by increasing endothelial layer permeability and expression of adhesion molecules by endothelial cells and leukocytes, activation of neutrophil and eosinophil functional activity, induction of acute phase reactants and other liver proteins, as well as tissue degrading enzymes produced by synoviocytes and/or chondrocytes. Cells expressing transmembrane TNFα bound by infliximab can be lysed in vitro or in vivo. Infliximab inhibits the functional activity of TNFα in a wide variety of in vitro bioassays utilizing human fibroblasts, endothelial cells, neutrophils, B and T-lymphocytes and epithelial cells. The relationship of these biological response markers to the mechanism(s) by which Infliximab exerts its clinical effects is unknown. Anti-TNFα antibodies reduce disease activity in the cotton-top tamarin colitis model, and decrease synovitis and joint erosions in a murine model of collagen-induced arthritis. Infliximab prevents disease in transgenic mice that develop polyarthritis as a result of constitutive expression of human TNFα, and when administered after disease onset, allows eroded joints to heal.
12.2 Pharmacodynamics
Elevated concentrations of TNFα have been found in involved tissues and fluids of patients with RA, CD, UC, AS, PsA, and Ps. In RA, treatment with Infliximab reduced infiltration of inflammatory cells into inflamed areas of the joint as well as expression of molecules mediating cellular adhesion [E-selectin, intercellular adhesion molecule-1 (ICAM-1) and vascular cell adhesion molecule-1 (VCAM-1)], chemoattraction [IL-8 and monocyte chemotactic protein (MCP-1)] and tissue degradation [matrix metalloproteinase (MMP) 1 and 3]. In CD, treatment with Infliximab reduced infiltration of inflammatory cells and TNFα production in inflamed areas of the intestine, and reduced the proportion of mononuclear cells from the lamina propria able to express TNFα and interferon. After treatment with Infliximab, patients with RA or CD exhibited decreased levels of serum IL-6 and C-reactive protein (CRP) compared to baseline. Peripheral blood lymphocytes from Infliximab-treated patients showed no significant decrease in number or in proliferative responses to in vitro mitogenic stimulation when compared to cells from untreated patients. In PsA, treatment with Infliximab resulted in a reduction in the number of T-cells and blood vessels in the synovium and psoriatic skin lesions as well as a reduction of macrophages in the synovium. In Ps, Infliximab treatment may reduce the epidermal thickness and infiltration of inflammatory cells. The relationship between these pharmacodynamic activities and the mechanism(s) by which Infliximab exerts its clinical effects is unknown.
12.3 Pharmacokinetics
In adults, single intravenous (IV) infusions of 3 mg/kg to 20 mg/kg (two times the maximum recommended dose for any indication) showed a linear relationship between the dose administered and the maximum serum concentration. The volume of distribution at steady state was independent of dose and indicated that infliximab was distributed primarily within the vascular compartment. Pharmacokinetic results for single doses of 3 mg/kg to 10 mg/kg in RA, 5 mg/kg in CD, and 3 mg/kg to 5 mg/kg in Ps indicate that the median terminal half- life of infliximab is 7.7 to 9.5 days.
Following an initial dose of Infliximab, repeated infusions at 2 and 6 weeks resulted in predictable concentration-time profiles following each treatment. No systemic accumulation of infliximab occurred upon continued repeated treatment with 3 mg/kg or 10 mg/kg at 4- or 8-week intervals. Development of antibodies to infliximab increased infliximab clearance. At 8 weeks after a maintenance dose of 3 to 10 mg/kg of Infliximab, median infliximab serum concentrations ranged from approximately 0.5 to 6 mcg/mL; however, infliximab concentrations were not detectable (<0.1 mcg/mL) in patients who became positive for antibodies to infliximab. No major differences in clearance or volume of distribution were observed in patient subgroups defined by age, weight, or gender. It is not known if there are differences in clearance or volume of distribution in patients with marked impairment of hepatic or renal function.
Infliximab pharmacokinetic characteristics (including peak and trough concentrations and terminal half-life) were similar in pediatric (aged 6 to 17 years) and adult patients with CD or UC following the administration of 5 mg/kg of Infliximab.
REFERENCES SECTION
15 REFERENCES
- Turner D, Otley AR, Mack D, et al. Development, validation, and evaluation of a pediatric ulcerative colitis activity index: A prospective multicenter study. Gastroenterology. 2007;133:423–432.
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
For injection: 100 mg of infliximab as a white lyophilized powder in a single- dose vial for reconstitution and dilution.
For injection: 100 mg of infliximab as a lyophilized powder in a single-dose vial for reconstitution and dilution. (2.11, 3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available observational studies in pregnant women exposed to Infliximab showed no increased risk of major malformations among live births as compared to those exposed to non-biologics. However, findings on other birth and maternal outcomes were not consistent across studies of different study design and conduct (see Data).
Monoclonal antibodies such as infliximab are transferred across the placenta during the third trimester of pregnancy and may affect immune response in the in utero exposed infant (see Clinical Considerations). Because infliximab does not cross-react with TNFα in species other than humans and chimpanzees, animal reproduction studies have not been conducted with Infliximab. In a developmental study conducted in mice using an analogous antibody, no evidence of maternal toxicity or fetal harm was observed (see Data).
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes.
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Clinical Considerations
Disease-Associated Maternal and/or Embryo/Fetal Risk
Published data suggest that there is an increased risk of adverse pregnancy outcomes in women with inflammatory bowel disease or rheumatoid arthritis associated with increased disease activity. Adverse pregnancy outcomes include preterm delivery (before 37 weeks of gestation), low birth weight (less than 2.5 kg) and small for gestational age at birth.
Fetal/Neonatal Adverse Reactions
As with other IgG antibodies, infliximab crosses the placenta. Infliximab has been detected in the serum of infants up to 6 months following birth. Consequently, these infants may be at increased risk of infection, including disseminated infection which can become fatal. At least a six month waiting period following birth is recommended before the administration of live vaccines (e.g., BCG vaccine or other live vaccines, such as the rotavirus vaccine) to these infants [see Warnings and Precautions (5.13)]. Cases of agranulocytosis in infants exposed in utero have also been reported [see Adverse Reactions (6.3)].
Data
Human Data
Two prospective cohort studies were conducted assessing birth outcomes as well as the health status of infants up to the age of one year in women exposed to Infliximab compared to non-biologic comparators including methotrexate, azathioprine, 6-mercaptopurine and systemic corticosteroids used for the treatment of similar diseases. The first study was conducted in an IBD pregnancy registry in the United States and assessed pregnancy outcomes in 294 women with inflammatory bowel disease exposed to Infliximab during pregnancy compared with 515 women on a non-biologic treatment. Infliximab exposure was not associated with increased rates of major congenital malformations, miscarriage/stillbirth, infants of low birth weight, small for gestational age, or infection in the first year of life. The second study among IBD and non-IBD patients in Sweden, Finland, and Denmark compared 97, 7, and 166 women exposed to Infliximab to 2,693, 2,499 and 1,268 women on non-biologic systemic therapy, respectively. In this study, comparing pooled data across the three countries, exposure to Infliximab was not associated with increased rates of congenital anomalies or infant death. Infliximab in combination with immunosuppressants (mainly systemic corticosteroids and azathioprine) was associated with increased rates of preterm birth, small for gestational age, low birth weight, and infant hospitalization for infection compared with non- biologic systemic treatment. Although the study did not show any associations with Infliximab monotherapy, the analyses could have been underpowered to detect an association.
There were additional methodological limitations with these studies that may account for the study findings in both studies: the concomitant use of other medications or treatments was not controlled and disease severity was not assessed; in the U.S. study, patient reported outcomes were collected without clinical validation. These methodological limitations hinder interpretation of the study results.
Animal Data
Because infliximab products do not cross-react with TNFα in species other than humans and chimpanzees, animal reproduction studies have not been conducted with infliximab. An embryofetal development study was conducted in pregnant mice using cV1q anti-mouse TNFα, an analogous antibody that selectively inhibits the functional activity of mouse TNFα. This antibody administered in mice, during the period of organogenesis on gestation days (GDs) 6 and 12, at IV doses up to 40 mg/kg produced no evidence of maternal toxicity, fetal mortality, or structural abnormalities. Doses of 10 to 15 mg/kg in pharmacodynamic animal models with the anti-TNF analogous antibody produced maximal pharmacologic effectiveness. Analyses of fetal samples on GD 14 indicated placental transfer of the antibody and exposure of the fetuses during organogenesis. In a peri- and post-natal development study in mice, no maternal toxicity or adverse developmental effects in offspring were observed when dams were administered IV doses of 10 or 40 mg/kg of the analogous antibody on GDs 6, 12 and 18 and lactation days 3, 9 and 15.
8.2 Lactation
Risk Summary
Published literature show that infliximab is present at low levels in human milk. Systemic exposure in a breastfed infant is expected to be low because infliximab is largely degraded in the gastrointestinal tract. A U.S. multi- center study of 168 women treated with infliximab for inflammatory bowel disease (breast milk samples obtained, n=29) showed that infants exposed to infliximab through breast milk had no increase in rates of infections and developed normally. There are no data on the effects of infliximab on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Infliximab and any potential adverse effects on the breastfed child from Infliximab or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of Infliximab have been established in pediatric patients 6 to 17 years of age for induction and maintenance treatment of CD and UC [see Dosage and Administration (2.2, 2.4) and Adverse Reactions (6.1)]. However, the safety and effectiveness of Infliximab in pediatric patients <6 years of age with CD or UC have not been established. The safety and effectiveness of Infliximab in the treatment of pediatric patients with Ps and juvenile rheumatoid arthritis (JRA) have not been established.
Pediatric Crohn's Disease
The safety and effectiveness of Infliximab have been established for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients 6 years of age and older with moderately to severely active CD who have had an inadequate response to conventional therapy. The use of Infliximab for this indication is supported by evidence from a randomized, open-label pediatric CD study in 112 pediatric patients aged 6 years and older [see Clinical Studies (14.2)].
Infliximab has been studied only in combination with conventional immunosuppressive therapy in pediatric CD. The longer term (greater than 1 year) safety and effectiveness of Infliximab in pediatric CD patients have not been established in clinical trials.
Postmarketing cases of HSTCL have been reported in pediatric patients treated with TNF blockers including Infliximab. Due to the risk of HSTCL, a careful risk-benefit assessment should be made when Infliximab is used in combination with other immunosuppressants in pediatric CD patients [see Boxed Warning, Warnings and Precautions (5.2)].
Pediatric Ulcerative Colitis
The safety and effectiveness of Infliximab for reducing signs and symptoms and inducing and maintaining clinical remission in pediatric patients aged 6 years and older with moderately to severely active UC who have had an inadequate response to conventional therapy have been established. The use of Infliximab for this indication is supported by evidence from adequate and well-controlled studies of Infliximab in adults with additional safety and pharmacokinetic data from an open-label pediatric UC study in 60 pediatric patients aged 6 years and older [see Dosage and Administration (2.4), Adverse Reactions (6.1), and Clinical Studies (14.4)]. The effectiveness of Infliximab in inducing and maintaining mucosal healing in pediatric UC was not established. Although 41 patients had a Mayo endoscopy subscore of 0 or 1 at the Week 8 endoscopy, the induction phase was open-label and lacked a control group. Only 9 patients had an optional endoscopy at Week 54. Approximately half of the patients were on concomitant immunomodulators (AZA, 6-MP, MTX) at study start.
Due to the risk of HSTCL, a careful risk-benefit assessment should be made when Infliximab is used in combination with other immunosuppressants in pediatric UC patients [see Boxed Warning and Warnings and Precautions (5.2)].
The longer term (greater than 1 year) safety and effectiveness of Infliximab in pediatric UC patients have not been established in clinical trials.
Juvenile Rheumatoid Arthritis (JRA)
The safety and effectiveness of Infliximab in the treatment of pediatric patients with juvenile rheumatoid arthritis (JRA) havenot been established.
The safety and efficacy of Infliximab in patients with JRA were evaluated in a multicenter, randomized, placebo-controlled, double-blind study for 14 weeks, followed by a double-blind, all-active treatment extension, for a maximum of 44 weeks. Patients with active JRA between the ages of 4 and 17 years who had been treated with MTX for at least 3 months were enrolled. Concurrent use of folic acid, oral corticosteroids (≤0.2 mg/kg/day of prednisone or equivalent), NSAIDs, and/or disease modifying antirheumatic drugs (DMARDs) was permitted.
Doses of 3 mg/kg Infliximab or placebo were administered intravenously at Weeks 0, 2 and 6. Patients randomized to placebo crossed-over to receive 6 mg/kg Infliximab at Weeks 14, 16, and 20, and then every 8 weeks through Week 44. Patients who completed the study continued to receive open-label treatment with Infliximab for up to 2 years in a companion extension study.
The study failed to establish the efficacy of Infliximab in the treatment of JRA. Key observations in the study included a high placebo response rate and a higher rate of immunogenicity than what has been observed in adults. Additionally, a higher rate of clearance of infliximab was observed than had been observed in adults.
Population pharmacokinetic analysis showed that in pediatric patients with JRA with a body weight of up to 35 kg receiving 6 mg/kg Infliximab and pediatric patients with JRA with body weight greater than 35 kg up to adult body weight receiving 3 mg/kg Infliximab, the steady state area under the concentration curve (AUCss) was similar to that observed in adults receiving 3 mg/kg of Infliximab.
A total of 60 patients with JRA were treated with doses of 3 mg/kg and 57 patients were treated with doses of 6 mg/kg. The proportion of patients with infusion reactions who received 3 mg/kg Infliximab was 35% (21/60) over 52 weeks compared with 18% (10/57) in patients who received 6 mg/kg over 38 weeks. The most common infusion reactions reported were vomiting, fever, headache, and hypotension. In the 3 mg/kg Infliximab group, 4 patients had a serious infusion reaction and 3 patients reported a possible anaphylactic reaction (2 of which were among the serious infusion reactions). In the 6 mg/kg Infliximab group, 2 patients had a serious infusion reaction, 1 of whom had a possible anaphylactic reaction. Two of the 6 patients who experienced serious infusion reactions received Infliximab by rapid infusion (duration of less than 2 hours). Antibodies to infliximab developed in 38% (20/53) of patients who received 3 mg/kg Infliximab compared with 12% (6/49) of patients who received 6 mg/kg.
A total of 68% (41/60) of patients who received 3 mg/kg Infliximab in combination with MTX experienced an infection over 52 weeks compared with 65% (37/57) of patients who received 6 mg/kg Infliximab in combination with MTX over 38 weeks. The most commonly reported infections were upper respiratory tract infection and pharyngitis, and the most commonly reported serious infection was pneumonia. Other notable infections included primary varicella infection in 1 patient and herpes zoster in 1 patient.
8.5 Geriatric Use
Of the total number of Infliximab-treated patients in RA and Ps clinical studies, 256 (9.6%) were 65 years old and over, while 17 (0.6%) were 75 years old and over. In these trials, no overall differences in safety or effectiveness were observed between geriatric patients (patients ≥ 65 years old) and younger adult patients (patients 18 to 65 years old). However, the incidence of serious adverse reactions in geriatric patients was higher in both Infliximab and control groups compared to younger adult patients.
Of the total number of Infliximab-treated patients in CD, UC, AS, and PsA clinical studies, 76 (3.2%) were 65 years old and over, while 9 (0.4%) were 75 years old and over. In the CD, UC, AS, and PsA studies, there were insufficient numbers of geriatric patients to determine whether they respond differently from younger adults .
The incidence of serious infections in Infliximab-treated geriatric patients was greater than in Infliximab-treated younger adult patients; therefore close monitoring of geriatric patients for the development of serious infections is recommended [see Warnings and Precautions (5.1), and Adverse Reactions (6.1)].
OVERDOSAGE SECTION
10 OVERDOSAGE
Single doses up to 20 mg/kg have been administered without any direct toxic effect. In case of overdosage, it is recommended that the patient be monitored for any signs or symptoms of adverse reactions or effects [see Warnings and Precautions (5)] and appropriate symptomatic treatment instituted immediately.
DESCRIPTION SECTION
11 DESCRIPTION
Infliximab, a tumor necrosis factor (TNF) blocker, is a chimeric IgG1κ monoclonal antibody (composed of human constant and murine variable regions). It has a molecular weight of approximately 149.1 kilodaltons. Infliximab is produced by a recombinant murine myeloma cell line, SP2/0.
Infliximab for injection is supplied as a sterile, preservative-free, white, lyophilized powder for intravenous infusion after reconstitution and dilution. Following reconstitution with 10 mL of Sterile Water for Injection, USP, the final concentration is 10 mg/mL and the resulting pH is approximately 7.2. Each single-dose vial contains 100 mg infliximab, dibasic sodium phosphate, dihydrate (6.1 mg), monobasic sodium phosphate, monohydrate (2.2 mg), polysorbate 80 (0.5 mg), and sucrose (500 mg).
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
A 6-month study in CD-1 mice was conducted to assess the tumorigenic potential of cV1q anti-mouse TNFα, an analogous antibody. No evidence of tumorigenicity was observed in mice that received intravenous doses of 10 mg/kg or 40 mg/kg cV1q given weekly. The relevance of this study for human risk is unknown. No impairment of fertility or reproductive performance indices were observed in male or female mice that received cV1q, an analogous mouse antibody, at intravenous doses up to 40 mg/kg given weekly.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Infliximab for injection is supplied in a carton containing one single-dose vial (NDC 57894-160-01).
Each single-dose vial contains 100 mg of infliximab as a sterile, preservative-free, white lyophilized powder for reconstitution and dilution (more than one vial may be needed for a full dose) [see Dosage and Administration (2.11)].
Storage and Handling
Store unopened Infliximab vials in a refrigerator at 2°C to 8°C (36°F to 46°F).
If needed, unopened Infliximab vials may be stored at room temperatures up to a maximum of 30°C (86°F) for a single period of up to 6 months but not exceeding the original expiration date. The new expiration date must be written in the space provided on the carton. Once removed from the refrigerator, Infliximab cannot be returned to the refrigerator.
For storage conditions of the reconstituted and diluted product for administration, see Dosage and Administration (2.11).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient or their caregiver to read the FDA-approved patient labeling (Medication Guide).
Patients or their caregivers should be advised of the potential benefits and risks of Infliximab. Healthcare providers should instruct their patients or their caregivers to read the Medication Guide before starting Infliximab therapy and to reread it each time they receive an infusion.
Infections
Inform patients that Infliximab increases the risk for developing serious infections. Instruct patients of the importance of contacting their healthcare provider if they develop any symptoms of an infection, including tuberculosis, invasive fungal infections, and reactivation of hepatitis B virus infections [see Warnings and Precautions (5.1, 5.3)].
Malignancies
Malignancies have been reported among children, adolescents and young adults who received treatment with TNF blockers. Patients should be counseled about the risk of lymphoma and other malignancies while receiving Infliximab [see Warnings and Precautions (5.2)].
Hepatotoxicity
Instruct patients to seek medical attention if they develop signs or symptoms of hepatotoxicity (e.g., jaundice) [see Warnings and Precautions (5.4)].
Heart Failure
Instruct patients to seek medical attention and consult their prescriber if they develop signs or symptoms of heart failure [see Contraindications (4) and Warnings and Precautions (5.5)].
Hematologic Reactions
Instruct patients to seek immediate medical attention if they develop signs and symptoms suggestive of blood dyscrasias or infection (e.g., persistent fever) while on Infliximab [see Warnings and Precautions (5.6)].
Hypersensitivity
Advise patients to seek immediate medical attention if they experience any symptoms of serious hypersensitivity reactions [see Warnings and Precautions (5.7)].
Cardiovascular and Cerebrovascular Reactions During and After Infusion
Advise patients to seek immediate medical attention if they develop any new or worsening symptoms of cardiovascular and cerebrovascular reactions which have been reported during and within 24 hours of initiation of Infliximab infusion [see Warnings and Precautions (5.8)].
Neurologic Reactions
Advise patients to seek medical attention if they develop signs or symptoms of neurologic reactions [see Warnings and Precautions (5.9)].
Live Vaccines/Therapeutic Infectious Agents
Instruct Infliximab-treated patients to avoid receiving live vaccines or therapeutic infectious agents [see Warnings and Precautions (5.13)].
SPL UNCLASSIFIED SECTION
Manufactured by:
Janssen Biotech, Inc.
Horsham, PA 19044
U.S. License No. 1864
SPL MEDGUIDE SECTION
|
MEDICATION GUIDE | |||
|---|---|---|---|
|
This Medication Guide has been approved by the U.S. Food and Drug Administration |
Revised: October 2021 | ||
|
Read the Medication Guide that comes with Infliximab before you receive the first treatment, and before each time you get a treatment of Infliximab. This Medication Guide does not take the place of talking with your doctor about your medical condition or treatment. | |||
|
What is the most important information I should know about Infliximab? | |||
|
Infliximab may cause serious side effects, including: | |||
|
1. Risk of infection | |||
|
Infliximab is a medicine that affects your immune system. Infliximab can lower the ability of your immune system to fight infections. Serious infections have happened in patients receiving Infliximab. These infections include tuberculosis (TB) and infections caused by viruses, fungi or bacteria that have spread throughout the body. Some patients have died from these infections. | |||
| |||
|
Before starting Infliximab, tell your doctor if you: | |||
| |||
|
After starting Infliximab, if you have an infection, any sign of an infection including a fever, cough, flu-like symptoms, or have open cuts or sores on your body, call your doctor right away. Infliximab can make you more likely to get infections or make any infection that you have worse. | |||
|
2. Risk of Cancer | |||
| |||
|
See the section "What are the possible side effects of Infliximab?" below for more information. | |||
|
What is Infliximab? | |||
|
Infliximab is a prescription medicine that is approved for patients with: | |||
| |||
|
Infliximab blocks the action of a protein in your body called tumor necrosis factor-alpha (TNF-alpha). TNF-alpha is made by your body's immune system. People with certain diseases have too much TNF-alpha that can cause the immune system to attack normal healthy parts of the body. Infliximab can block the damage caused by too much TNF-alpha. | |||
|
It is not known if Infliximab is safe and effective in children under 6 years of age. | |||
|
Who should not receive Infliximab? | |||
|
You should not receive Infliximab if you have: | |||
| |||
|
What should I tell my doctor before starting treatment with Infliximab? | |||
|
Your doctor will assess your health before each treatment. | |||
|
Tell your doctor about all of your medical conditions, including if you: | |||
| |||
|
If you have a baby and you were receiving Infliximab during your pregnancy, it is important to tell your baby's doctor and other health care professionals about your Infliximab use so they can decide when your baby should receive any vaccine. Certain vaccinations can cause infections. | |||
|
If you received Infliximab while you were pregnant, your baby may be at higher risk for getting an infection. If your baby receives a live vaccine within 6 months after birth, your baby may develop infections with serious complications that can lead to death. This includes live vaccines such as the BCG, rotavirus, or any other live vaccines. For other types of vaccines, talk with your doctor. | |||
|
How should I receive Infliximab? | |||
| |||
|
What should I avoid while receiving Infliximab? | |||
|
Do not take Infliximab together with medicines such as KINERET (anakinra), ORENCIA (abatacept), ACTEMRA (tocilizumab), or other medicines called biologics that are used to treat the same conditions as Infliximab. | |||
|
Tell your doctor about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. These include any other medicines to treat Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriatic arthritis or psoriasis. | |||
|
Know the medicines you take. Keep a list of your medicines and show them to your doctor and pharmacist when you get a new medicine. | |||
|
What are the possible side effects of Infliximab? | |||
|
Infliximab can cause serious side effects, including: | |||
|
See "What is the most important information I should know about Infliximab?" | |||
|
Serious Infections | |||
| |||
|
| ||
| |||
|
| ||
|
Heart Failure | |||
|
If you have a heart problem called congestive heart failure, your doctor should check you closely while you are receiving Infliximab. Your congestive heart failure may get worse while you are receiving Infliximab. Be sure to tell your doctor of any new or worse symptoms including: | |||
|
| ||
|
Treatment with Infliximab may need to be stopped if you get new or worse congestive heart failure. | |||
|
Other Heart Problems | |||
|
Some patients have experienced a heart attack (some of which led to death), low blood flow to the heart, or abnormal heart rhythm within 24 hours of beginning their infusion of Infliximab. Symptoms may include chest discomfort or pain, arm pain, stomach pain, shortness of breath, anxiety, lightheadedness, dizziness, fainting, sweating, nausea, vomiting, fluttering or pounding in your chest, and/or a fast or a slow heartbeat. Tell your doctor right away if you have any of these symptoms. | |||
|
Liver Injury | |||
|
Some patients receiving Infliximab have developed serious liver problems. Tell your doctor if you have: | |||
|
| ||
|
Blood Problems | |||
|
In some patients receiving Infliximab, the body may not make enough of the blood cells that help fight infections or help stop bleeding. Tell your doctor if you: | |||
|
| ||
|
Nervous System Disorders | |||
|
Some patients receiving Infliximab have developed problems with their nervous system. Tell your doctor if you have: | |||
|
| ||
|
Some patients have experienced a stroke within approximately 24 hours of their infusion with Infliximab. Tell your doctor right away if you have symptoms of a stroke which may include: numbness or weakness of the face, arm or leg, especially on one side of the body; sudden confusion, trouble speaking or understanding; sudden trouble seeing in one or both eyes, sudden trouble walking, dizziness, loss of balance or coordination or a sudden, severe headache. | |||
|
Allergic Reactions | |||
|
Some patients have had allergic reactions to Infliximab. Some of these reactions were severe. These reactions can happen while you are getting your Infliximab treatment or shortly afterward. Your doctor may need to stop or pause your treatment with Infliximab and may give you medicines to treat the allergic reaction. Signs of an allergic reaction can include: | |||
|
| ||
|
Some patients treated with Infliximab have had delayed allergic reactions. The delayed reactions occurred 3 to 12 days after receiving treatment with Infliximab. Tell your doctor right away if you have any of these signs of delayed allergic reaction to Infliximab: | |||
|
| ||
|
Lupus-like Syndrome | |||
|
Some patients have developed symptoms that are like the symptoms of Lupus. If you develop any of the following symptoms, your doctor may decide to stop your treatment with Infliximab. | |||
|
| ||
|
Psoriasis | |||
|
Some people receiving Infliximab had new psoriasis or worsening of psoriasis they already had. Tell your doctor if you develop red scaly patches or raised bumps on the skin that are filled with pus. Your doctor may decide to stop your treatment with Infliximab. | |||
|
The most common side effects of Infliximab include: | |||
|
| ||
|
Infusion reactions can happen up to 2 hours after your infusion of Infliximab. Symptoms of infusion reactions may include: | |||
|
|
| |
|
Children who received Infliximab in studies for Crohn's disease showed some differences in side effects compared with adults who received Infliximab for Crohn's disease. The side effects that happened more in children were: anemia (low red blood cells), leukopenia (low white blood cells), flushing (redness or blushing), viral infections, neutropenia (low neutrophils, the white blood cells that fight infection), bone fracture, bacterial infection and allergic reactions of the breathing tract. Among patients who received Infliximab for ulcerative colitis in clinical studies, more children had infections as compared with adults. | |||
|
Tell your doctor about any side effect that bothers you or does not go away. | |||
|
These are not all of the side effects with Infliximab. Ask your doctor or pharmacist for more information. | |||
|
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |||
|
General information about Infliximab | |||
|
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. | |||
|
You can ask your doctor or pharmacist for information about Infliximab that is written for health professionals. | |||
|
For more information call 1-800-JANSSEN (1-800-526-7736). | |||
|
What are the ingredients in Infliximab? | |||
|
The active ingredient is Infliximab. | |||
|
The inactive ingredients in Infliximab include: dibasic sodium phosphate dihydrate, monobasic sodium phosphate monohydrate, polysorbate 80, and sucrose. No preservatives are present. | |||
|
Manufactured by: Janssen Biotech, Inc. Horsham, PA 19044 |
