ColdCalm Kids
ColdCalm Kids Liquid
6b3d8ff0-065d-85c4-e053-2991aa0a1788
HUMAN OTC DRUG LABEL
Sep 2, 2025
Boiron
DUNS: 282560473
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
STRYCHNOS NUX-VOMICA SEED, PULSATILLA VULGARIS, ONION, PHYTOLACCA AMERICANA ROOT, POTASSIUM DICHROMATE, GELSEMIUM SEMPERVIRENS ROOT, EUPATORIUM PERFOLIATUM FLOWERING TOP, APIS MELLIFERA,
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL


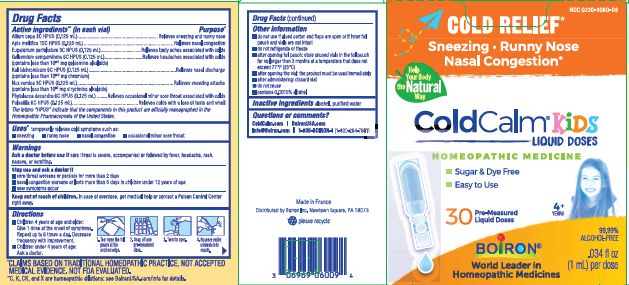

INDICATIONS & USAGE SECTION
Uses*
Temporarily relieves cold symptoms such as:
- sneezing
- runny nose
- nasal congestion
- occasional minor sore throat
OTC - QUESTIONS SECTION
ColdCalm.com
** BoironUSA.com**
** info@boiron.com**
** 1-800-BOIRON-1**(1-800-264-7661)
Distributed by Boiron Inc. Newtown Square, PA 19073-3267
INACTIVE INGREDIENT SECTION
alcohol, purified water
OTC - ACTIVE INGREDIENT SECTION
Active ingredients (in each 1 mL vial)**
Allium cepa 3C HPUS (0.125 mL)
Apis mellifica 15C HPUS (0.125 mL)
Eupatorium perfoliatum 3C HPUS (0.125 mL)
Gelsemium sempervirens 6C HPUS (0.125 mL) (contains less than 10 -11 mg gelsemine alkaloids)
Kali bichromicum 6C HPUS (0.125 mL) (contains less than 10 -10 mg chromium)
Nux vomica 3C HPUS (0.125 mL) (contains less than 10 -5 strychnine alkaloids)
Phytolacca decandra 6C HPUS (0.125 mL)
Pulsatilla 6C HPUS (0.125 mL)
The letters "HPUS" indicate that the components in this product are officially monographed in the Homeopathic Pharmacopoeia of the United States.
OTC - PURPOSE SECTION
Purpose*
Allium cepa 3C HPUS (0.125 mL) ... Relieves sneezing and runny nose
Apis mellifica 15C HPUS (0.125 mL) ... Relieves nasal congestion
Eupatorium perfoliatum 3C HPUS (0.125 mL) ... Relieves body aches associated with colds
Gelsemium sempervirens 6C HPUS (0.125 mL) ...Relieves headaches associated with colds
Kali bichromicum 6C HPUS (0.125 mL) ... Relieves nasal discharge
Nux vomica 3C HPUS (0.125 mL) ... Relieves sneezing attacks
Phytolacca decandra 6C HPUS (0.125 mL) ... Relieves occasional minor sore throat associated with colds
Pulsatilla 6C HPUS (0.125 mL) ... Relieves colds with a loss of taste and smell
WARNINGS SECTION
OTC - ASK DOCTOR SECTION
Ask a doctor before use ifsore throat is severe, accompanied or follow by fever, headache, rash, nausea, or vomiting.
OTC - STOP USE SECTION
Stop use and ask a doctor if
- sore throat worsens or persists for more than 2 days
- nasal congestion worsens or lasts more than 5 days in children under 12 years of age
- new symptoms occur
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Children 4 years of age and older: Give 1 dose at the onset of symptoms. Repeat up to 6 times a day. Decrease frequency with improvement.
Children under 4 years of age: Ask a doctor.
- Tear open the foil pouch at the notched edge.
- Snap off pre-measured dose.
- Twist to open.
- Holding the child upright, squeeze entire contents into mouth.
SPL UNCLASSIFIED SECTION
- do not use if glued carton end flaps are open or if inner foil pouch and vials are not intact
- do not refrigerate or freeze
- after opening foil pouch: store unused vials in the foil pouch and in the box for no longer than 3 months
- after opening the vial: use product immediately then discard
- contains 0.0015% alcohol
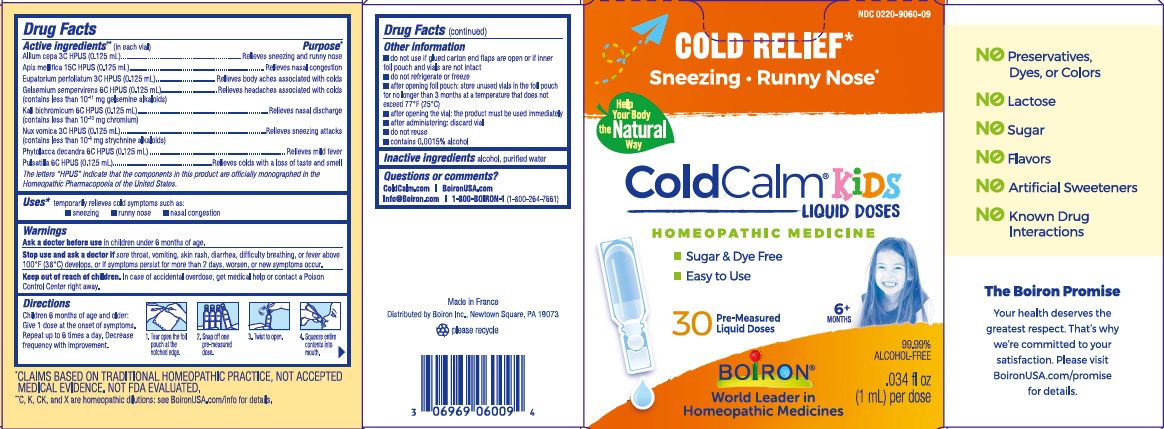
30 Doses
.034 fl oz (1 mL) per dose
No Known Drug Interactions
Cold Relief*
Sneezy, Runny Nose, Nasal Congestion*
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
**C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.
