iShancare NAIL FUNGUS SERUM
361a61cd-150c-5fcc-e063-6394a90ad553
HUMAN OTC DRUG LABEL
Jan 1, 2025
Shenzhen Ishan Technology Co., Ltd
DUNS: 554484192
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
NAIL FUNGUS SERUM
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Lable
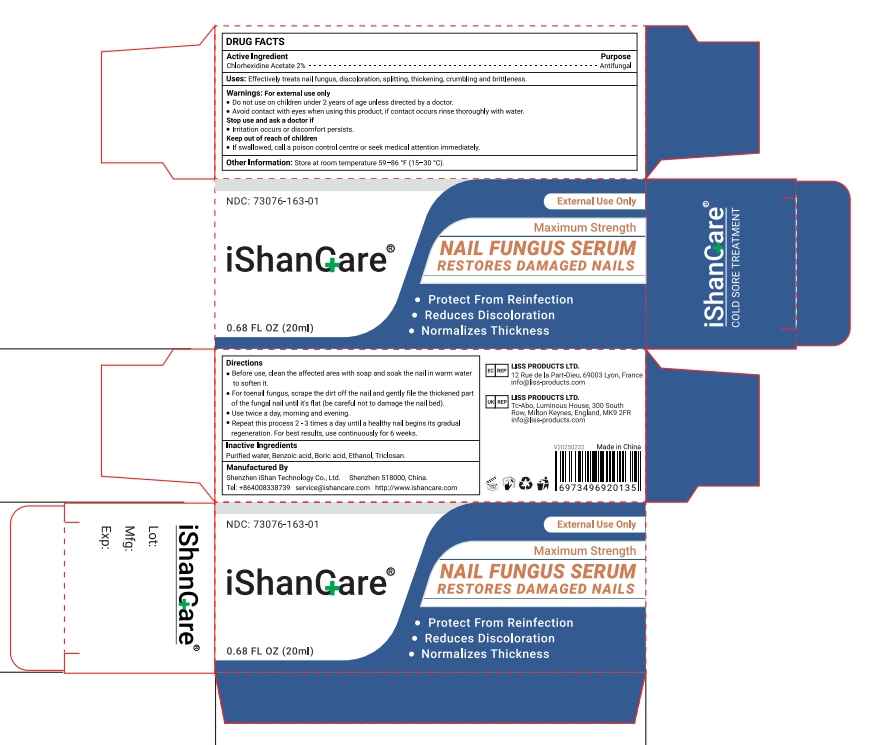
INDICATIONS & USAGE SECTION
Directions
-
Before use, clean the affected area with soap and soak the nail in warm waterto soften it.
-
For toenail fungus, scrape the dirt off the nail and gently file the thickened partof the fungal nail until it's flat (be careful not to damage the nail bed).
-
Use twice a day, morning and evening.
-
Repeat this process 2-3 times a day until a healthy nail begins its gradualregeneration.
-
For best results, use continuously for 6 weeks.
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Chlorhexidine Acetate 2%
INACTIVE INGREDIENT SECTION
Inactive ingredient
Purified water, Benzoic acid, Boric acid, Ethanol, Triclosan.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children.
if swallowed, call poison control or seek medical help.
OTC - ASK DOCTOR/PHARMACIST SECTION
Ask a doctor if
OTC - STOP USE SECTION
Stop use and ask a doctor if
pain worsens or persists, or if youexperience any of these symptoms: burning, itching, rash, or other changes in theskin where the product was placed.
OTC - PURPOSE SECTION
USES
Effectively treats nail fungus, discoloration, splitting, thickening, crumbling and brittleness.
STORAGE AND HANDLING SECTION
Other information
Store at room temperature 59-86°F(15-30°C)
DOSAGE & ADMINISTRATION SECTION
Dosage & administration
Apply 2-3 times a day
USER SAFETY WARNINGS SECTION
When using this product
Do not wear this product directly on skin.
Be aware of low-temperature burns.
WARNINGS SECTION
Warning
Warnings: For external use only
-
Do not use on children under 2 years of age unless directed by a doctor.
-
Avoid contact with eyes when using this product, if contact occurs rinse thoroughly with water
-
Stop use and ask a doctor if
lrritation occurs or discomfort persists.Keep out of reach of children -
If swallowed, call a poison control centre or seek medical attention immediately.
