Abib Watery hyaluron sunscreen Moisture tube
33d35125-eb1c-beef-e063-6394a90a2470
HUMAN OTC DRUG LABEL
Aug 27, 2025
Fourcompany Co., Ltd.
DUNS: 694864584
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Avobenzone, Homosalate, Octisalate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (35)
Drug Labeling Information
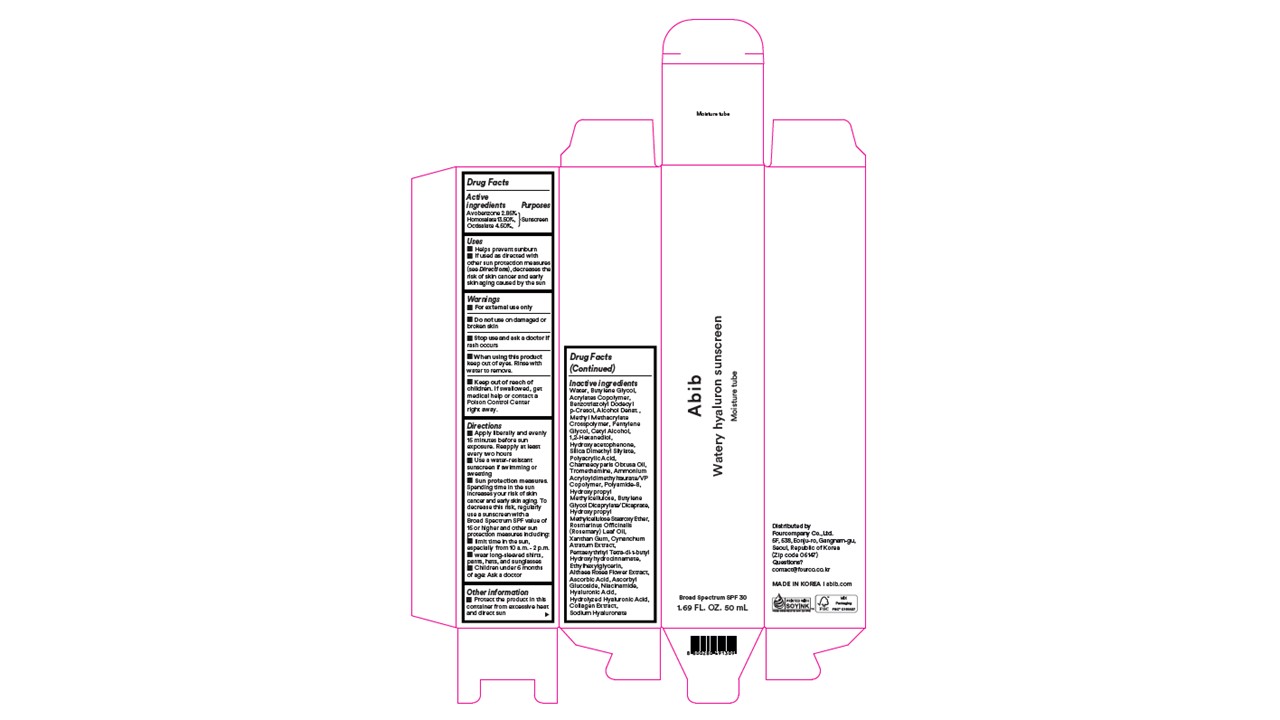
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Abib
Watery hyaluron sunscreen
Moisture tube
Broad Spectrum SPF 30
1.69 FL. OZ. 50 mL
INDICATIONS & USAGE SECTION
Uses
- Helps prevent sunburn
- If used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Avobenzone 2.85%,
Homosalate 13.50%,
Octisalate 4.50%
OTC - DO NOT USE SECTION
Do not use
Do not use on damaged or broken skin
OTC - STOP USE SECTION
Stop use
Stop use and ask a doctor if rash occurs
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away.
OTC - PURPOSE SECTION
Uses
Helps prevent sunburn
If used as directed with other sun protection measures (see Directions),
decreases the risk of skin cancer and early skin aging caused by the sun
OTC - WHEN USING SECTION
When using
For external use only
When using this product keep out of eyes. Rinse with water to remove.
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Water, Butylene Glycol, Acrylates Copolymer, Benzotriazolyl Dodecyl p-Cresol, Alcohol Denat., Methyl Methacrylate Crosspolymer, Pentylene Glycol, Cetyl Alcohol, 1,2-Hexanediol, Hydroxyacetophenone, Silica Dimethyl Silylate, Polyacrylic Acid, Chamaecyparis Obtusa Oil, Tromethamine, Ammonium Acryloyldimethyltaurate/VP Copolymer, Polyamide-8, Hydroxypropyl Methylcellulose, Butylene Glycol Dicaprylate/Dicaprate, Hydroxypropyl Methylcellulose Stearoxy Ether, Rosmarinus Officinalis (Rosemary) Leaf Oil, Xanthan Gum, Cynanchum Atratum Extract, Pentaerythrityl Tetra-di-t-butyl Hydroxyhydrocinnamate, Ethylhexylglycerin, Althaea Rosea Flower Extract, Ascorbic Acid, Ascorbyl Glucoside, Niacinamide, Hyaluronic Acid, Hydrolyzed Hyaluronic Acid, Collagen Extract, Sodium Hyaluronate
DOSAGE & ADMINISTRATION SECTION
Directions
1.Apply liberally and evenly 15 minutes before sun exposure. Reapply at least every two hours
2.Use a water-resistant sunscreen if swimming or sweating
3.Sun protection measures.
Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk,
regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
■ limit time in the sun, especially from 10 a.m. - 2 p.m.
■ wear long-sleeved shirts, pants, hats, and sunglasses
6.Children under 6 months of age: Ask a doctor
WARNINGS SECTION
Warnings
- For external use only
- Do not use on damaged or broken skin
- Stop use and ask a doctor if rash occurs
- When using this product keep out of eyes. Rinse with water to remove.
