Prolensa
These highlights do not include all the information needed to use PROLENSA safely and effectively. See full prescribing information for PROLENSA ophthalmic solution.PROLENSA (bromfenac ophthalmic solution), for topical ophthalmic use Initial U.S. Approval: 1997
4e072537-f73c-4a96-a65f-e2805ce112d8
HUMAN PRESCRIPTION DRUG LABEL
Jan 31, 2023
Bausch & Lomb Incorporated
DUNS: 196603781
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Bromfenac Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Sulfite Allergic Reactions
PROLENSA ophthalmic solution contains sodium sulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in non-asthmatic people.
5.2 Slow or Delayed Healing
All topical nonsteroidal anti-inflammatory drugs (NSAIDs), including bromfenac, may slow or delay healing. Topical corticosteroids are also known to slow or delay healing. Concomitant use of topical NSAIDs and topical steroids may increase the potential for healing problems.
5.3 Potential for Cross-Sensitivity
There is the potential for cross-sensitivity to acetylsalicylic acid, phenylacetic acid derivatives, and other NSAIDs, including bromfenac. Therefore, caution should be used when treating individuals who have previously exhibited sensitivities to these drugs.
5.4 Increased Bleeding Time
With some NSAIDs, including bromfenac, there exists the potential for increased bleeding time due to interference with platelet aggregation. There have been reports that ocularly applied NSAIDs may cause increased bleeding of ocular tissues (including hyphemas) in conjunction with ocular surgery.
It is recommended that PROLENSA ophthalmic solution be used with caution in patients with known bleeding tendencies or who are receiving other medications which may prolong bleeding time.
5.5 Keratitis and Corneal Reactions
Use of topical NSAIDs may result in keratitis. In some susceptible patients, continued use of topical NSAIDs may result in epithelial breakdown, corneal thinning, corneal erosion, corneal ulceration or corneal perforation. These events may be sight threatening. Patients with evidence of corneal epithelial breakdown should immediately discontinue use of topical NSAIDs, including bromfenac, and should be closely monitored for corneal health.
Postmarketing experience with topical NSAIDs suggests that patients with complicated ocular surgeries, corneal denervation, corneal epithelial defects, diabetes mellitus, ocular surface diseases (e.g., dry eye syndrome), rheumatoid arthritis, or repeat ocular surgeries within a short period of time may be at increased risk for corneal adverse events which may become sight threatening. Topical NSAIDs should be used with caution in these patients.
Postmarketing experience with topical NSAIDs also suggests that use more than 24 hours prior to surgery or use beyond 14 days postsurgery may increase patient risk for the occurrence and severity of corneal adverse events.
5.6 Contact Lens Wear
PROLENSA should not be instilled while wearing contact lenses. Remove contact lenses prior to instillation of PROLENSA. The preservative in PROLENSA, benzalkonium chloride, may be absorbed by soft contact lenses. Lenses may be reinserted after 10 minutes following administration of PROLENSA.
• Sulfite Allergic Reactions (5.1)
• Slow or Delayed Healing (5.2)
• Potential for Cross-Sensitivity (5.3)
• Increased Bleeding Time (5.4)
• Keratitis and Corneal Reactions (5.5)
• Contact Lens Wear (5.6)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The most commonly reported adverse reactions following use of PROLENSA following cataract surgery include: anterior chamber inflammation, foreign body sensation, eye pain, photophobia, and blurred vision. These reactions were reported in 3% to 8% of patients.
The most commonly reported adverse reactions in 3% to 8% of patients were anterior chamber inflammation, foreign body sensation, eye pain, photophobia, and blurred vision. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Bausch & Lomb Incorporated at 1-800-553-5340 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosing
One drop of PROLENSA ophthalmic solution should be applied to the affected eye once daily beginning 1 day prior to cataract surgery, continued on the day of surgery, and through the first 14 days of the postoperative period.
2.2 Use with Other Topical Ophthalmic Medications
PROLENSA ophthalmic solution may be administered in conjunction with other topical ophthalmic medications such as alpha-agonists, beta-blockers, carbonic anhydrase inhibitors, cycloplegics, and mydriatics. Drops should be administered at least 5 minutes apart.
Instill one drop into the affected eye once daily beginning 1 day prior to surgery, continued on the day of surgery, and through the first 14 days postsurgery. (2.1)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Treatment of rats at oral doses up to 0.9 mg/kg/day (systemic exposure 90 times the systemic exposure predicted from the recommended human ophthalmic dose [RHOD] assuming the human systemic concentration is at the limit of quantification) and rabbits at oral doses up to 7.5 mg/kg/day (150 times the predicted human systemic exposure) produced no treatment-related malformations in reproduction studies. However, embryofetal lethality and maternal toxicity were produced in rats and rabbits at 0.9 mg/kg/day and 7.5 mg/kg/day, respectively. In rats, bromfenac treatment caused delayed parturition at 0.3 mg/kg/day (30 times the predicted human exposure), and caused dystocia, increased neonatal mortality, and reduced postnatal growth at 0.9 mg/kg/day.
There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Because of the known effects of prostaglandin biosynthesis-inhibiting drugs on the fetal cardiovascular system (closure of ductus arteriosus), the use of PROLENSA ophthalmic solution during late pregnancy should be avoided.
8.3 Nursing Mothers
Caution should be exercised when PROLENSA ophthalmic solution is administered to a nursing woman.
8.4 Pediatric Use
Safety and efficacy in pediatric patients below the age of 18 years have not been established.
8.5 Geriatric Use
There is no evidence that the efficacy or safety profiles for PROLENSA differ in patients 70 years of age and older compared to younger adult patients.
DESCRIPTION SECTION
11 DESCRIPTION
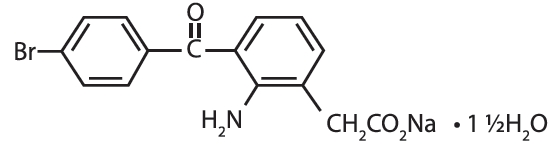
PROLENSA® (bromfenac ophthalmic solution) 0.07% is a sterile, topical, nonsteroidal anti-inflammatory drug (NSAID) for ophthalmic use. Each mL of PROLENSA contains 0.805 mg bromfenac sodium sesquihydrate (equivalent to 0.7 mg bromfenac free acid). The USAN name for bromfenac sodium sesquihydrate is bromfenac sodium. Bromfenac sodium is designated chemically as sodium [2-amino-3-(4-bromobenzoyl) phenyl] acetate sesquihydrate, with an empirical formula of C15H11BrNNaO3• 1½H2O. The chemical structure for bromfenac sodium sesquihydrate is:
Bromfenac sodium is a yellow to orange crystalline powder. The molecular weight of bromfenac sodium is 383.17.
PROLENSA ophthalmic solution is supplied as a sterile aqueous 0.07% solution, with a pH of 7.8. The osmolality of PROLENSA ophthalmic solution is approximately 300 mOsmol/kg.
Each mL of PROLENSA ophthalmic solution contains:
Active: Each mL contains bromfenac sodium sesquihydrate 0.0805%, which is equivalent to bromfenac free acid 0.07.
Inactives: boric acid, edetate disodium, povidone, sodium borate, sodium sulfite, tyloxapol, sodium hydroxide to adjust pH, and water for injection, USP.
Preservative: benzalkonium chloride 0.005%
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in rats and mice given oral doses of bromfenac up to 0.6 mg/kg/day (systemic exposure 30 times the systemic exposure predicted from the recommended human ophthalmic dose [RHOD] assuming the human systemic concentration is at the limit of quantification) and 5 mg/kg/day (340 times the predicted human systemic exposure), respectively, revealed no significant increases in tumor incidence.
Bromfenac did not show mutagenic potential in various mutagenicity studies, including the reverse mutation, chromosomal aberration, and micronucleus tests.
Bromfenac did not impair fertility when administered orally to male and female rats at doses up to 0.9 mg/kg/day and 0.3 mg/kg/day, respectively (systemic exposure 90 and 30 times the predicted human exposure, respectively).
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Ocular Inflammation and Pain
Bromfenac 0.07% QD for the treatment of postoperative inflammation and reduction of ocular pain was evaluated in two multi-center, randomized, double-masked, parallel-group, and placebo (vehicle)-controlled studies. Patients undergoing cataract surgery self-administered bromfenac 0.07% or vehicle once daily, beginning 1 day prior to surgery, continuing on the morning of surgery and for 14 days after surgery. Complete clearance of ocular inflammation (0 cell and no flare) was assessed on Days 1, 3, 8, and 15 postsurgery using slit lamp biomicroscopy. The pain score was self-reported. The primary efficacy endpoint was the proportion of subjects who had complete clearance of ocular inflammation by Day 15. In the intent-to-treat analyses from both assessments, complete clearance at Day 8 and Day 15, bromfenac 0.07% was superior to vehicle as shown in the following table.
|
Proportion of Subjects with Cleared Ocular Inflammation (0 cells and no flare) | ||||
|
Study |
Visit |
Bromfenac 0.07% |
Vehicle |
Difference (%) (Asymptotic 95% CI) |
|
Study 1 |
At Day 8 |
27/112 (24.1%) |
7/108 (6.5%) |
17.6 (8.4, 26.8) |
|
At Day 15 |
51/112 (45.5%) |
14/108 (13.0%) |
32.5 (21.4, 43.8) | |
|
Study 2 |
At Day 8 |
33/110 (30.0%) |
14/110 (12.7%) |
17.3 (6.7, 27.9) |
|
At Day 15 |
50/110 (45.5%) |
30/110 (27.3%) |
18.2 (5.7, 30.7) | |
|
Proportion of Subjects Who Were Pain Free | ||||
|
Study |
Visit |
Bromfenac 0.07% |
Vehicle |
Difference (%) (Asymptotic 95% CI) |
|
Study 1 |
At Day 1 |
91/112 (81.3%) |
47/108 (43.5%) |
37.7 (25.9, 49.6) |
|
Study 2 |
At Day 1 |
84/110 (76.4%) |
61/110 (55.5%) |
20.9 (8.7, 33.1) |
