Mannitol
HIGHLIGHTSOF PRESCRIBING INFORMATIONThese highlights do not include all the information needed to use MANNITOL INJECTION safely and effectively. See full prescribing information for MANNITOL INJECTION.MANNITOL injection, for intravenous useInitial U.S. Approval: 1964
e2444db1-9dfe-451f-a33c-9b00786464d2
HUMAN PRESCRIPTION DRUG LABEL
Nov 9, 2023
Henry Schein, Inc.
DUNS: 012430880
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Mannitol
PRODUCT DETAILS
INGREDIENTS (1)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Sample Package Label

ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions associated with the use mannitol were identified in clinical studies or postmarketing reports. Because some of these reactions were reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
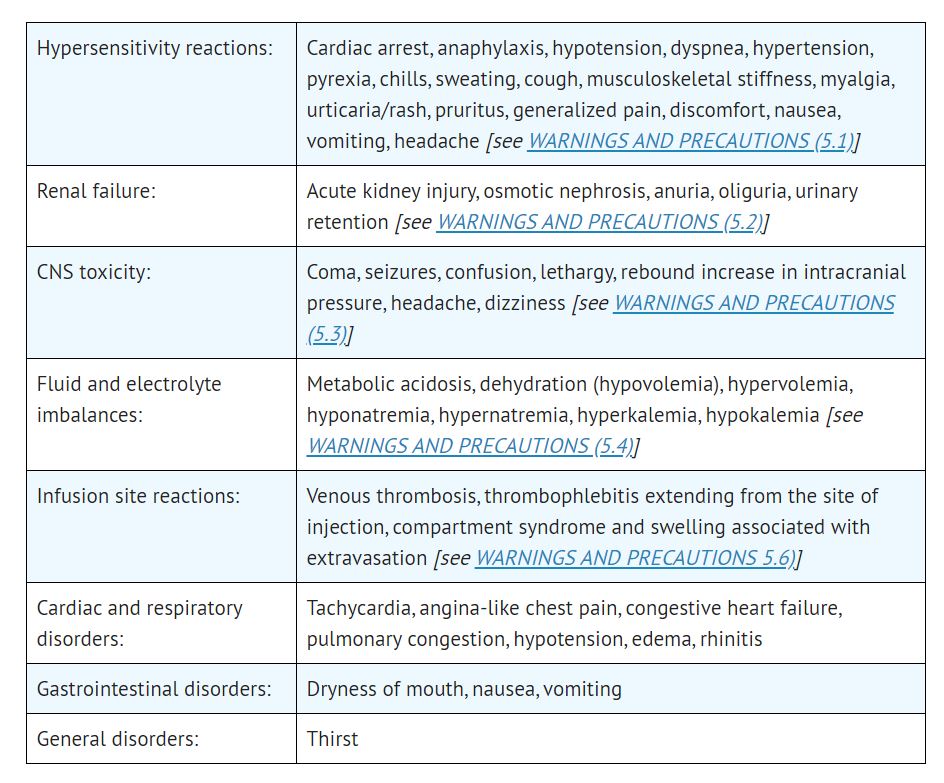
Most common adverse reactions are hypersensitivity reactions, renal failure, CNS toxicity, hypo/hypervolemia, hypo/hypernatremia, hypo/hyperkalemia, and infusion site reactions. (6) (7)
To report SUSPECTED ADVERSE REACTIONS, contact Hospira, Inc. at 1-800-441-4100 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (7)
DESCRIPTION SECTION
11 DESCRIPTION
Mannitol Injection, USP is a sterile, nonpyrogenic solution of mannitol in water for injection available in a fliptop vial for intravenous administration as an osmotic diuretic.
The content and characteristics are as follows:
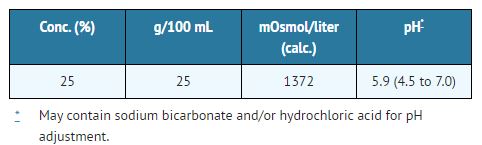
The solution contains no bacteriostat, antimicrobial agent, or added buffer (except for pH adjustment) and is intended only as a single-dose injection.
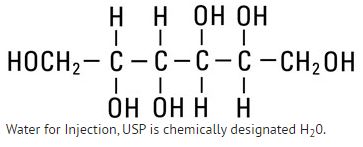
Mannitol, USP is chemically designated D-mannitol (C6H14O6), a white crystalline powder or free-flowing granules freely soluble in water. It has the following structural formula: