Trospium Chloride
These highlights do not include all the information needed to use Trospium Chloride Tablets safely and effectively. See full prescribing information for Trospium Chloride Tablets. TROSPIUM chloride tablets, USP for oral useInitial U.S. Approval:2004
570c3ae7-8011-4944-8935-57d0a66102aa
HUMAN PRESCRIPTION DRUG LABEL
Aug 21, 2023
Heritage Pharmaceuticals Inc. d/b/a Avet Pharmaceuticals Inc.
DUNS: 780779901
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Trospium Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (16)
Drug Labeling Information
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Trospium chloride was evaluated for the treatment of patients with overactive bladder who had symptoms of urinary frequency, urgency, and urge incontinence in two U.S. 12-week, placebo-controlled studies and one 9-month open label extension.
Study 1 was a randomized, double-blind, placebo-controlled, parallel-group study in 523 patients. A total of 262 patients received trospium chloride tablets 20 mg twice daily and 261 patients received placebo. The majority of patients were Caucasian (85%) and female (74%) with a mean age of 61 years (range: 21 to 90 years). Entry criteria required that patients have urge or mixed incontinence (with a predominance of urge), urge incontinence episodes of at least 7 per week, and greater than 70 micturitions per week. The patient's medical history and urinary diary during the treatment-free baseline confirmed the diagnosis. Reductions in urinary frequency, urge incontinence episodes and urinary void volume for placebo and trospium chloride treatment groups are summarized in Table 3 and Figures 2 and 3.
Table 3. Mean (SE) change from baseline to end of treatment (Week 12 or last observation carried forward) for urinary frequency, urge incontinence episodes, and void volume in Study 1|
** Efficacy endpoint** |
** Placebo**** N=256** |
** Trospium Chloride**** N=253** |
** P-value** |
|
** Urinary frequency/24 hours**a,* |
12.9 |
12.7 |
<0.001 |
|
** Urge incontinence episodes/week**b,* |
30.1 |
27.3 |
0.012 |
|
** Urinary void volume/toilet void (mL)**a,c |
156.6 |
155.1 |
<0.001 |
|
a Treatment differences assessed by analysis of variance for ITT:LOCF data
set.
|
Figure 2 – Mean Change from Baseline in Urinary Frequency/24 Hours, by Visit: Study 1
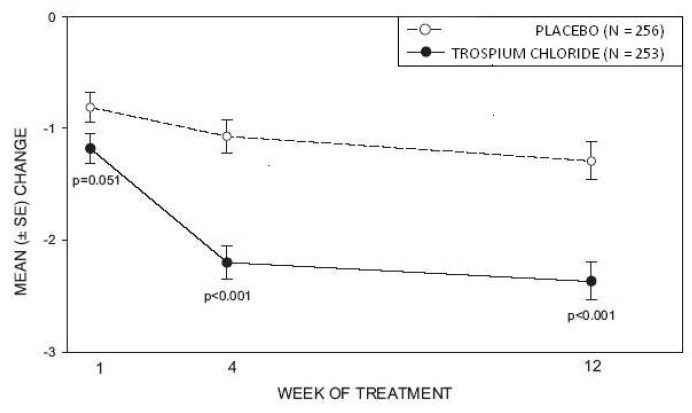
Figure 3 – Mean Change from Baseline in Urge Incontinence/Week, by Visit: Study 1
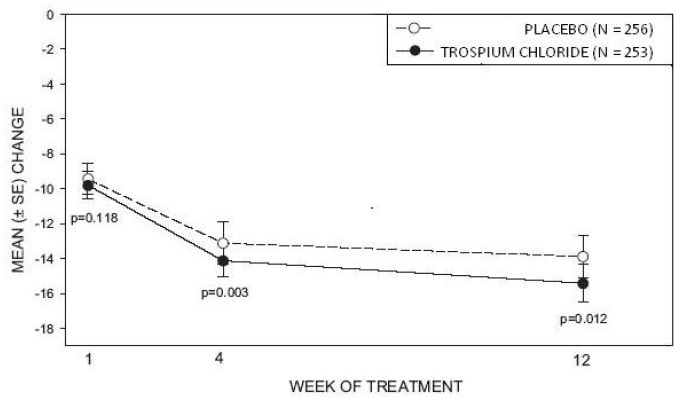
Study 2 was nearly identical in design to Study 1. A total of 329 patients received trospium chloride tablets 20 mg twice daily and 329 patients received placebo. The majority of patients were Caucasian (88%) and female (82%) with a mean age of 61 years (range: 19 to 94 years). Entry criteria were identical to Study 1. Reductions in urinary frequency, urge incontinence episodes, and urinary void volume for placebo and trospium chloride treatment groups are summarized in Table 4 and Figures 4 and 5.
Table 4. Mean (SE) change from baseline to end of treatment (Week 12 or last observation carried forward) for urinary frequency, urge incontinence episodes, and void volume in Study 2|
** Efficacy endpoint** |
** Placebo**** N=325** |
** Trospium** |
** P-value** |
|
** Urinary frequency/24 hours**a,* |
13.2 |
12.9 |
<0.001 |
|
** Urge incontinence episodes/week**b,* |
27.3 |
26.9 |
<0.001 |
|
** Urinary void volume/toilet void (mL)**a,c |
154.6 |
154.8 |
<0.001 |
|
a Treatment differences assessed by analysis of variance for ITT:LOCF data
set.
|
Figure 4 – Mean Change from Baseline in Urinary Frequency/24 Hours, by Visit: Study 2
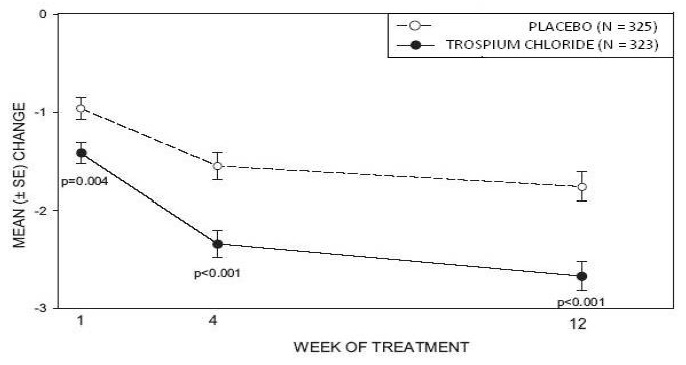
Figure 5 – Mean Change from Baseline in Urge Incontinence/Week, by Visit: Study 2
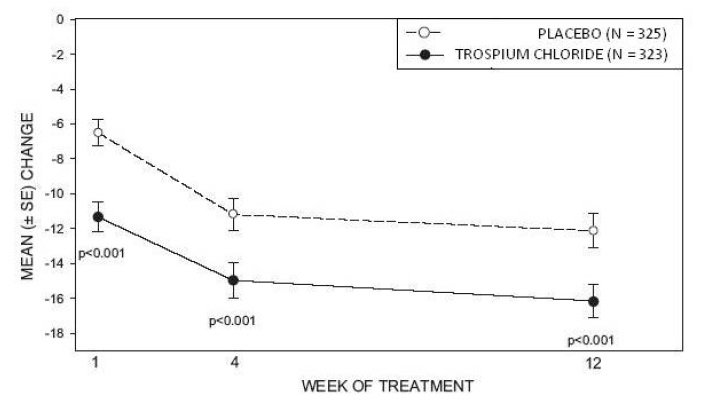
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
"See FDA-approved Patient Labeling (Patient Information)"
17.1 Angioedema
Patients should be informed that trospium chloride, the active ingredient in trospium chloride tablets, may produce angioedema which could result in life- threatening airway obstruction. Patients should be advised to promptly discontinue trospium chloride tablets and seek immediate medical attention if they experience edema of the tongue, edema of the laryngopharynx, or difficulty breathing.
17.2 When Not to Use
Prior to treatment, patients should fully understand the risks and benefits of trospium chloride. In particular, patients should be informed not to take trospium chloride tablets if they:
- have urinary retention;
- gastric retention;
- uncontrolled narrow-angle glaucoma;
- are allergic to any component of trospium chloride tablets.
17.3 Administration
Patients should be instructed regarding the recommended dosing and administration of trospium chloride tablets:
- Take one trospium chloride tablet twice daily with water.
- Take trospium chloride tablets on an empty stomach or at least 1 hour before a meal.
17.4 Adverse Reactions
Patients should be informed that the most common side effects with trospium chloride tablets are dry mouth and constipation and that other less common side effects include trouble emptying the bladder, blurred vision, and heat prostration. Because anticholinergics, such as trospium chloride, may produce dizziness or blurred vision, patients should be advised to exercise caution in decisions to engage in potentially dangerous activities until the drug's effects have been determined. Patients should be informed that alcohol may enhance the drowsiness caused by anticholinergic agents.
+SANCUTURA XR® is a registered trademark by Allergan Inc. for Trospium Chloride Extended-Release Capsules, 60 mg.
++Glucophage® is a registered trade mark by Bristol Meyers Squibb for Metformin Hydrochloride Tablets.
Distributed by:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

Revised: 10/2021
Patient Information Leaflet
Trospium (TROSE-pee-um) Chloride Tablets, USP
Read the Patient Information that comes with trospium chloride tablets before you start taking it and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your doctor about your medical condition or your treatment.
What is trospium chloride tablets?
Trospium chloride tablets are a prescription medicine used to treat adults with overactive bladder who have the following symptoms:
- a strong need to urinate right away;
- leaking or wetting accidents due to a strong need to urinate right away;
- a need to urinate often.
Who should not take trospium chloride tablets?
Do not take trospium chloride tablets if you:
- have trouble emptying your bladder;
- have delayed or slow emptying of your stomach;
- have an eye problem called "uncontrolled narrow-angle glaucoma";
- are allergic to trospium chloride tablets or any of its ingredients. See the end of this leaflet for a complete list of ingredients.
Trospium chloride tablets has not been studied in children under the age of 18 years.
What should I tell my doctor before starting trospium chloride tablets?
Tell your doctor about all of your medical conditions including if you:
- have any stomach or intestinal problems or problems with constipation;
- have trouble emptying your bladder or have a weak urine stream;
- have an eye problem called narrow-angle glaucoma;
- have kidney problems;
- have liver problems;
- are pregnant or planning to become pregnant. It is not known if trospium chloride can harm your unborn baby.
- are breastfeeding. It is not known if trospium chloride passes into breast milk and if it can harm your baby. You should talk to your doctor about the best way to feed your baby if you are taking trospium chloride tablets.
Tell your doctor about all the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements. Trospium chloride tablets and certain other medicines can interact and make some side effects worse. Trospium chloride tablets can affect how other medicines are handled by the body.
Know all the medicines you take. Keep a list of them with you to show your doctor and pharmacist each time you get a new medicine.
How should I take trospium chloride tablets?
Take trospium chloride tablets exactly as prescribed.
- Take one trospium chloride tablet twice daily with water.
- Take trospium chloride tablets on an empty stomach or at least 1 hour before a meal.
- If you take too much trospium chloride, call your local Poison Control Center or go to an emergency room right away.
What are the possible side effects of trospium chloride tablets?
Trospium chloride tablets may cause allergic reactions that may be serious. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat or tongue. If you experience these symptoms, you should stop taking trospium chloride tablets and get emergency medical help right away.
The most common side effects with trospium chloride tablets are:
- dry mouth;
- constipation;
- headache.
Trospium chloride tablets may cause other less common side effects, including:
- trouble emptying the bladder;
- blurred vision; and drowsiness. Do not drive or operate heavy machinery until you know how trospium chloride tablets affects you. Alcohol can worsen the drowsiness caused by drugs such as trospium chloride.
- heat prostration. Due to decreased sweating, heat prostration can occur when drugs such as trospium chloride are used in a hot environment.
Tell your doctor if you have any side effects that bother you or that do not go away.
These are not all possible side effects of trospium chloride. For more information, ask your doctor, healthcare professional or pharmacist.
How should I store trospium chloride tablets?
- Keep trospium chloride tablet and all other medicines out of the reach of children.
- Store trospium chloride tablet at room temperature, 68° to 77°F (20° to 25°C).
- Safely dispose of trospium chloride tablets that are out of date or that you no longer need.
General information about trospium chloride tablets
Medicines are sometimes prescribed for conditions that are not mentioned in patient information leaflets. Do not use trospium chloride tablet for a condition for which it was not prescribed. Do not give trospium chloride tablet to other people, even if they have the same symptoms you have. It may harm them.
This leaflet summarizes the most important information about trospium chloride tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about trospium chloride tablets that is written for health professionals. You can also call Avet at 1-866-901-DRUG (3784).
What are the ingredients in trospium chloride tablets?
Active Ingredient: trospium chloride.
Inactive Ingredients: black iron oxide, confectioners' sugar, croscarmellose sodium, hypromellose, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone, propylene glycol, shellac, silicon dioxide, talc and titanium dioxide.
Dispense with Patient Information available at: www.avetpharma.com/product
Distributed by:
Avet Pharmaceuticals Inc.
East Brunswick, NJ 08816
1.866.901.DRUG (3784)

51U000000240US06
Revised:10/2021
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions, Central Nervous System Effects (5.5) 07/2012
Warnings and Precautions, Central Nervous System Effects (5.5) 07/2012
