SupreH Bee Venom Skin Tag Treatment Liquid
83818-012
35ca9836-917a-b0ac-e063-6294a90a28a2
HUMAN OTC DRUG LABEL
May 23, 2025
Shenzhen Xinxin Yunhai Technology Co., Ltd.
DUNS: 699816806
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SupreH Bee Venom Skin Tag Remover Liquid
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
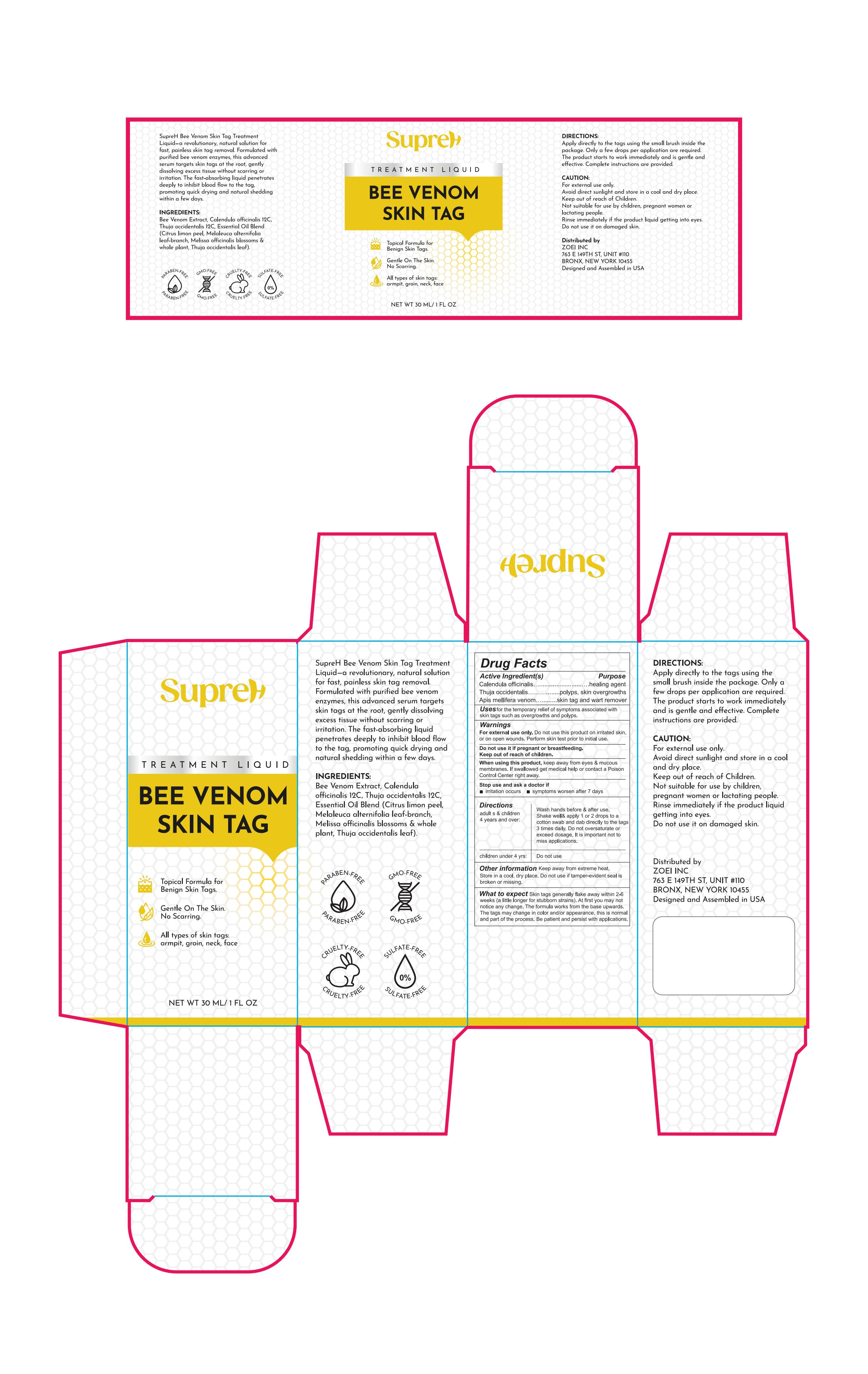
INDICATIONS & USAGE SECTION
Use
for the temporary relief of symptoms associated with skin tags such as overgrowths and polyps
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Calendula officinalis 2%
Thuja occidentalis 2%
Apis mellifera venom 2%
OTC - PURPOSE SECTION
Purpose
Calendula officinalis ……healing agent
Thuja occidentalis………polyps, skin overgrowths
Apis mellifera venom……skin tag and wart remover
WARNINGS SECTION
Warnings
For external use only. Do not use this product on irritated skin, or on open wounds. Perform skin test prior to initial use.
OTC - DO NOT USE SECTION
Do not use
Do not use it if pregnant or breastfeeding. Keep out of reach of children.
OTC - WHEN USING SECTION
When Using
When using this product, keep away from eyes & mucous membranes. If swallowed get medical help or contact a Poison Control Center right away.
OTC - STOP USE SECTION
Stop Use
Stop use and ask a doctor if
1.Irritation occurs
2.symptoms worsen after 7 days
OTC - ASK DOCTOR SECTION
Ask Doctor
Stop use and ask a doctor if
1.Irritation occurs
2.symptoms worsen after 7 days
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep Out Of Reach Of Children
Please keep out of reach of children. If children accidentally swallow it, please call the emergency center immediately.
DOSAGE & ADMINISTRATION SECTION
Directions
Adult s & children 4 years and over:
Wash hands before & after use. Shake well& apply 1 or 2 drops to a cotton swab
and dab directly to the tags 3 times daily. Do not oversaturate or exceed
dosage. It is important not to miss applications.
Children under 4 yrs: Do not use
STORAGE AND HANDLING SECTION
Other information
Keep away from extreme heat. Store in a cool, dry place. Do not use if tamper- evident seal is broken or missing.
INACTIVE INGREDIENT SECTION
Inactive ingredients
PHELLODENDRON CHINENSIS WHOLE
CITRUS LIMON (LEMON) PEEL OIL
MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL
MELISSA OFFICINALIS WHOLE
SWEET ALMOND OIL
SALICYLIC ACID
DESCRIPTION SECTION
What to expect
Skin tags generally flake away within 2-6 weeks (a little longer for stubborn strains). At first you may not notice any change. The formula works from the base upwards. The tags may change in color and/or appearance, this is normal and part of the process. Be patient and persist with applications.
