Dexmedetomidine
These highlights do not include all the information needed to use DEXMEDETOMIDINE INJECTION safely and effectively. See full prescribing information for DEXMEDETOMIDINE INJECTION. DEXMEDETOMIDINE injection, for intravenous use Initial U.S. Approval: 1999
81a1df0e-89b3-e13d-e053-2991aa0a4db4
HUMAN PRESCRIPTION DRUG LABEL
May 16, 2023
Medical Purchasing Solutions, LLC.
DUNS: 601458529
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
dexmedetomidine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (3)
Drug Labeling Information
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Drug Administration
Dexmedetomidine should be administered only by persons skilled in the management of patients in the operating room setting. Due to the known pharmacological effects of dexmedetomidine, patients should be continuously monitored while receiving dexmedetomidine.
5.2 Hypotension, Bradycardia, and Sinus Arrest
Clinically significant episodes of bradycardia and sinus arrest have been reported with dexmedetomidine administration in young, healthy adult volunteers with high vagal tone or with different routes of administration including rapid intravenous or bolus administration.
Reports of hypotension and bradycardia have been associated with dexmedetomidine infusion. Some of these cases have resulted in fatalities. If medical intervention is required, treatment may include decreasing or stopping the infusion of dexmedetomidine, increasing the rate of intravenous fluid administration, elevation of the lower extremities, and use of pressor agents. Because dexmedetomidine has the potential to augment bradycardia induced by vagal stimuli, clinicians should be prepared to intervene. The intravenous administration of anticholinergic agents (e.g., glycopyrrolate, atropine) should be considered to modify vagal tone. In clinical trials, glycopyrrolate or atropine were effective in the treatment of most episodes of dexmedetomidine-induced bradycardia. However, in some patients with significant cardiovascular dysfunction, more advanced resuscitative measures were required.
Caution should be exercised when administering dexmedetomidine to patients with advanced heart block and/or severe ventricular dysfunction. Because dexmedetomidine decreases sympathetic nervous system activity, hypotension and/or bradycardia may be expected to be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension and in elderly patients.
In clinical trials where other vasodilators or negative chronotropic agents were co-administered with dexmedetomidine an additive pharmacodynamic effect was not observed. Nonetheless, caution should be used when such agents are administered concomitantly with dexmedetomidine.
5.3 Transient Hypertension
Transient hypertension has been observed primarily during the loading dose in association with the initial peripheral vasoconstrictive effects of dexmedetomidine. Treatment of the transient hypertension has generally not been necessary, although reduction of the loading infusion rate may be desirable.
5.4 Arousability
Some patients receiving dexmedetomidine have been observed to be arousable and alert when stimulated. This alone should not be considered as evidence of lack of efficacy in the absence of other clinical signs and symptoms.
5.5 Withdrawal
Procedural Sedation
In adult subjects, withdrawal symptoms were not seen after discontinuation of short term infusions of dexmedetomidine (<6 hours).
5.6 Tolerance and Tachyphylaxis
Use of dexmedetomidine beyond 24 hours has been associated with tolerance and tachyphylaxis and a dose-related increase in adverse reactions [see Adverse Reactions ( 6.1)] .
5.7 Hepatic Impairment
Since dexmedetomidine clearance decreases with severity of hepatic impairment, dose reduction should be considered in patients with impaired hepatic function [see Dosage and Administration ( 2.2)] .
- Monitoring: Continuously monitor patients while receiving dexmedetomidine. ( 5.1)
- Bradycardia and Sinus Arrest: Have occurred in young healthy volunteers with high vagal tone or with different routes of administration, e.g., rapid intravenous or bolus administration. ( 5.2)
- Hypotension and Bradycardia: May necessitate medical intervention. May be more pronounced in patients with hypovolemia, diabetes mellitus, or chronic hypertension, and in the elderly. Use with caution in patients with advanced heart block or severe ventricular dysfunction. ( 5.2)
- Co-administration with Other Vasodilators or Negative Chronotropic Agents: Use with caution due to additive pharmacodynamic effects. ( 5.2)
- Transient Hypertension: Observed primarily during the loading dose. Consider reduction in loading infusion rate. ( 5.3)
- Arousability: Patients can become aroused/alert with stimulation; this alone should not be considered as lack of efficacy. ( 5.4)
- Prolonged exposure to dexmedetomidine beyond 24 hours may be associated with tolerance and tachyphylaxis and a dose-related increase in adverse events. ( 5.6)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Anesthetics, Sedatives, Hypnotics, Opioids
Co-administration of dexmedetomidine with anesthetics, sedatives, hypnotics, and opioids is likely to lead to an enhancement of effects. Specific studies have confirmed these effects with sevoflurane, isoflurane, propofol, alfentanil, and midazolam. No pharmacokinetic interactions between dexmedetomidine and isoflurane, propofol, alfentanil and midazolam have been demonstrated. However, due to possible pharmacodynamic interactions, when co- administered with dexmedetomidine, a reduction in dosage of dexmedetomidine or the concomitant anesthetic, sedative, hypnotic or opioid may be required.
7.2 Neuromuscular Blockers
In one study of 10 healthy adult volunteers, administration of dexmedetomidine for 45 minutes at a plasma concentration of one ng/mL resulted in no clinically meaningful increases in the magnitude of neuromuscular blockade associated with rocuronium administration.
Anesthetics, Sedatives, Hypnotics, Opioids: Enhancement of pharmacodynamic effects. Reduction in dosage of dexmedetomidine or the concomitant medication may be required. ( 7.1)
DESCRIPTION SECTION
11 DESCRIPTION
Dexmedetomidine Injection, USP is a sterile, nonpyrogenic solution suitable for intravenous infusion following dilution. Dexmedetomidine hydrochloride is the S-enantiomer of medetomidine and is chemically described as (+)-4-(S)-[1-(2,3-dimethylphenyl)ethyl]-1H-imidazole monohydrochloride. Dexmedetomidine hydrochloride has a molecular weight of 236.7 and the empirical formula is C 13H 16N 2•HCl and the structural formula is:
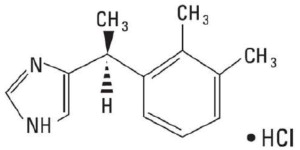
Dexmedetomidine hydrochloride is a white or almost white powder that is freely soluble in water and has a pKa of 7.1. Its partition coefficient in-octanol: water at pH 7.4 is 2.89.
Dexmedetomidine Injection, USP is supplied as a clear, colorless, isotonic solution with a pH of 4.5 to 7.0. Each mL contains 118 mcg of dexmedetomidine hydrochloride equivalent to 100 mcg (0.1 mg) of dexmedetomidine and 9 mg of sodium chloride in water and is to be used after dilution. The solution is preservative-free and contains no additives or chemical stabilizers.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Animal carcinogenicity studies have not been performed with dexmedetomidine.
Dexmedetomidine was not mutagenic in vitro, in either the bacterial reverse mutation assay ( E. coli and Salmonella typhimurium) or the mammalian cell forward mutation assay (mouse lymphoma). Dexmedetomidine was clastogenic in the in vitro human lymphocyte chromosome aberration test with, but not without, rat S9 metabolic activation. In contrast, dexmedetomidine was not clastogenic in the in vitro human lymphocyte chromosome aberration test with or without human S9 metabolic activation. Although dexmedetomidine was clastogenic in an in vivo mouse micronucleus test in NMRI mice, there was no evidence of clastogenicity in CD-1 mice.
Fertility in male or female rats was not affected after daily subcutaneous injections of dexmedetomidine at doses up to 54 mcg/kg (less than the maximum recommended human intravenous dose on a mcg/m 2 basis) administered from 10 weeks prior to mating in males, and 3 weeks prior to mating and during mating in females.
13.2 Animal Pharmacology and/or Toxicology
There were no differences in the adrenocorticotropic hormone (ACTH)-stimulated cortisol response in dogs following a single dose of dexmedetomidine compared to saline control. However, after continuous subcutaneous infusions of dexmedetomidine at 3 mcg/kg/hr and 10 mcg/kg/hr for one week in dogs (exposures estimated to be within the clinical range), the ACTH-stimulated cortisol response was diminished by approximately 27% and 40%, respectively, compared to saline-treated control animals indicating a dose-dependent adrenal suppression.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Dexmedetomidine Injection, USP is supplied as follows:
|
NDC |
Dexmedetomidine Injection, USP (100 mcg per mL) |
Package Factor |
|
70860-605-02 |
200 mcg per 2 mL Single-Dose Vial |
10 vials per carton |
|
70860-605-03 |
200 mcg per 2 mL Single-Dose Vial |
25 vials per carton |
Dexmedetomidine Injection, USP is available in clear glass vials. The strength is based on the dexmedetomidine base.
Storage Conditions
Store at 25°C (77°F); excursions permitted between 15° and 30°C (59° and 86°F). [See USP Controlled Room Temperature.]
Discard unused portion.
Sterile, Nonpyrogenic, Preservative-free.
The container closure is not made with natural rubber latex.
