Buspirone Hydrochloride
Buspirone Hydrochloride Tablets, USP(Patient Instruction Sheet Included)
cb0091ad-97f6-431b-b2b9-e03171eed274
HUMAN PRESCRIPTION DRUG LABEL
Mar 30, 2023
Strides Pharma Inc.
DUNS: 078310501
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Buspirone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Buspirone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Buspirone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Buspirone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Buspirone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

5mg-500-container

7.5mg-100-container

10mg-500-container

15mg-180-container
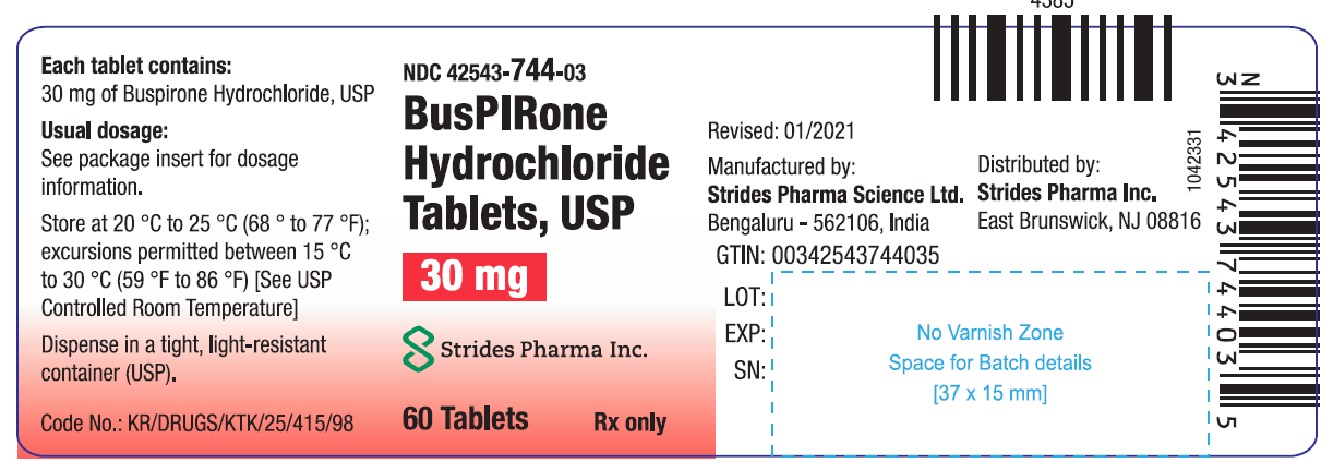
30mg-60-container
DESCRIPTION SECTION
DESCRIPTION
Buspirone hydrochloride is an antianxiety agent that is not chemically or pharmacologically related to the benzodiazepines, barbiturates, or other sedative/anxiolytic drugs.
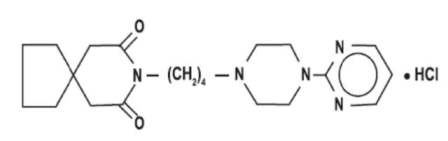
Buspirone hydrochloride is a white crystalline, water soluble compound with a molecular weight of 422.0. Chemically, buspirone hydrochloride is 8-[4-[4-(2-pyrimidinyl)-1-piperazinyl]butyl]-8-azaspiro[4,5]decane-7,9-dione monohydrochloride. The empirical formula C21H31N5O2.HCl is represented by the following structural formula:
Buspirone hydrochloride tablets, USP for oral administration, contains 5 mg, 7.5 mg, 10 mg, 15 mg or 30 mg of buspirone hydrochloride, USP (equivalent to 4.6 mg, 6.9 mg, 9.1 mg, 13.7 mg and 27.4 mg of buspirone free base respectively). The 5 mg and 10 mg tablets are scored so they can be bisected. Thus, the 5 mg tablet can also provide a 2.5 mg dose, and the 10 mg tablet can provide a 5 mg dose. The 15 mg tablets are scored such that they may be bisected or trisected. Thus, a single 15 mg tablet can provide the following doses: 15 mg (entire tablet), 10 mg (two thirds of a tablet), 7.5 mg (one-half of a tablet), or 5 mg (one-third of a tablet). The 30 mg tablets are scored such that they may be bisected or trisected. A single 30 mg tablet can provide the following doses: 30 mg (entire tablet), 20 mg (two-thirds of a tablet), 15 mg (one-half of a tablet), or 10 mg (one-third of a tablet). Buspirone hydrochloride tablets, USP contain the following inactive ingredients: colloidal silicon dioxide, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and sodium starch glycolate.
OVERDOSAGE SECTION
OVERDOSAGE
Signs and Symptoms
In clinical pharmacology trials, doses as high as 375 mg/day were administered to healthy male volunteers. As this dose was approached, the following symptoms were observed: nausea, vomiting, dizziness, drowsiness, miosis, and gastric distress. A few cases of overdosage have been reported, with complete recovery as the usual outcome. No deaths have been reported following overdosage with buspirone alone. Rare cases of intentional overdosage with a fatal outcome were invariably associated with ingestion of multiple drugs and/or alcohol, and a causal relationship to buspirone could not be determined. Toxicology studies of buspirone yielded the following LD50 values: mice, 655 mg/kg; rats, 196 mg/kg; dogs, 586 mg/kg; and monkeys, 356 mg/kg. These dosages are 160 to 550 times the recommended human daily dose.
Recommended Overdose Treatment
General symptomatic and supportive measures should be used along with immediate gastric lavage. Respiration, pulse, and blood pressure should be monitored as in all cases of drug overdosage. No specific antidote is known to buspirone, and dialyzability of buspirone has not been determined.
HOW SUPPLIED SECTION
HOW SUPPLIED
Buspirone Hydrochloride Tablets, USP are available as:
|
100 tablets in a HDPE bottle |
NDC: 42543-741-06 |
White to off white ovoid- rectangular uncoated functionally scored tablet with score line on one side and engraved '5' on the other side. | |
|
5 mg |
500 tablets in a HDPE bottle |
NDC: 42543-741-07 | |
|
1000 tablets in a HDPE bottle |
NDC: 42543-741-08 | ||
|
7.5 mg |
100 tablets in a HDPE |
NDC: 42543-787-06 |
White to off white colour, oval, biconvex tablets, debossed with 7.5 on one side and score line on other side. |
|
500 tablets in a HDPE |
NDC: 42543-787-07 | ||
|
100 tablets in a HDPE bottle |
NDC: 42543-742-06 |
White to off white ovoid- rectangular uncoated functionally scored tablet with score line on one side and engraved '10' on the other side. | |
|
10 mg |
500 tablets in a HDPE bottle |
NDC: 42543-742-07 | |
|
1000 tablets in a HDPE bottle |
NDC: 42543-742-08 | ||
|
60 tablets in a HDPE bottle |
NDC: 42543-743-03 |
White to off white rectangular uncoated functionally scored tablet with bisected score lines on one side and trisected score line with engraved '5' on each trisection of other side. The 15 mg tablet is in xx tablet design and functionally scored so that it can be either bisected or trisected. | |
|
15 mg |
100 tablets in a HDPE bottle |
NDC: 42543-743-06 | |
|
180 tablets in a HDPE bottle |
NDC: 42543-743-18 | ||
|
30 mg |
60 tablets in a HDPE bottle |
NDC: 42543-744-03 |
White to off white rectangular uncoated functionally scored tablet with bisected score lines on one side and trisected score line with engraved '10' on each trisection of other side. |
Store at 20 °C to 25 °C (68 °F to 77 °F); excursions permitted between 15 °C to 30 °C (59 °F to 86 °F) [See USP controlled room temperature]. Dispense in a tight, light-resistant container (USP).
INFORMATION FOR PATIENTS SECTION
BUSPIRONE HYDROCHLORIDE TABLETS, USP
PATIENT INFORMATION
Rx only
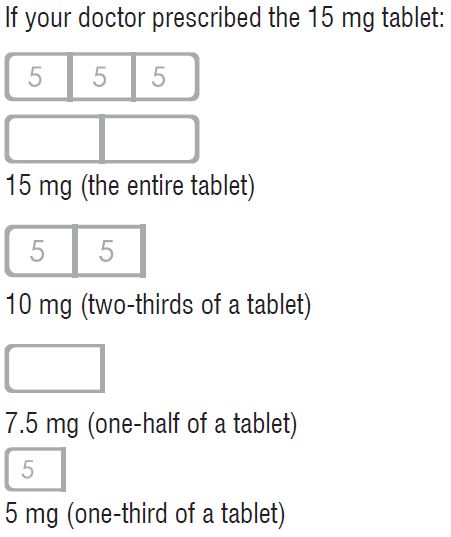
HOW TO USE BUSPIRONE HYDROCHLORIDE TABLETS, 15 mg
Response to buspirone varies among individuals. Your physician may find it necessary to adjust your dosage to obtain the proper response.
This tablet design makes dosage adjustments easy. Each tablet is scored and can be broken accurately to provide any of the following dosages:
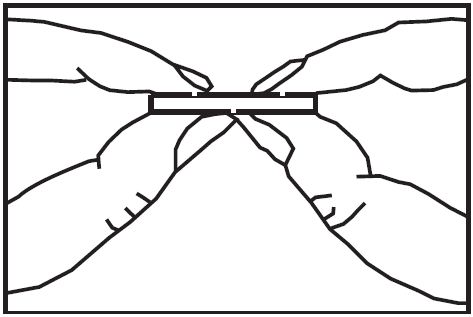
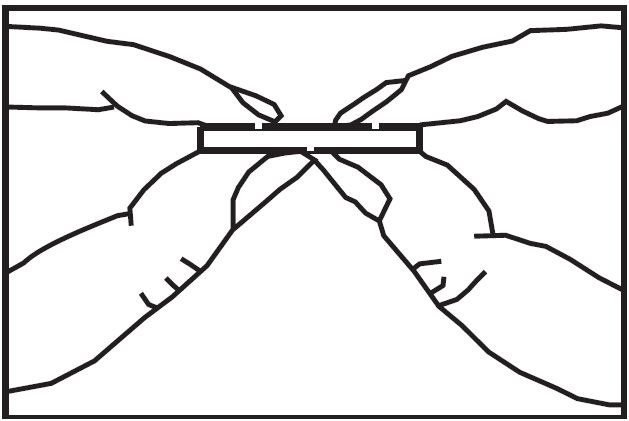
To break a tablet accurately and easily, hold the tablet between your thumbs and index fingers close to the appropriate tablet score (groove) as shown below. Then, with the tablet score facing you, apply pressure and snap the tablet segments apart (segments breaking incorrectly should not be used).
HOW TO USE BUSPIRONE HYDROCHLORIDE TABLETS, 30 mg
Response to buspirone varies among individuals. Your physician may find it necessary to adjust your dosage to obtain the proper response.
This tablet design makes dosage adjustments easy. Each tablet is scored and can be broken accurately to provide any of the following dosages:
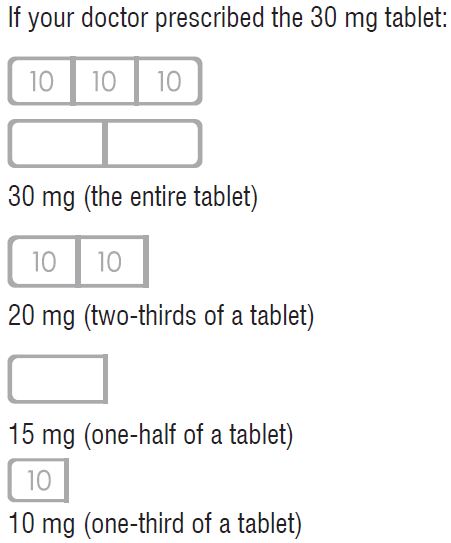
To break a tablet accurately and easily, hold the tablet between your thumbs and index fingers close to the appropriate tablet score (groove) as shown below. Then, with the tablet score facing you, apply pressure and snap the tablet segments apart (segments breaking incorrectly should not be used).
Distributed by:
Strides Pharma Inc.
East Brunswick, NJ 08816
Revised: 01/2021
