allergy relief
Equaline 44-585 Delisted
e34d8bf5-e12e-453c-93ea-9c7988c4f13d
HUMAN OTC DRUG LABEL
Sep 16, 2025
United Natural Foods, Inc. dba UNFI
DUNS: 943556183
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Diphenhydramine HCl
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
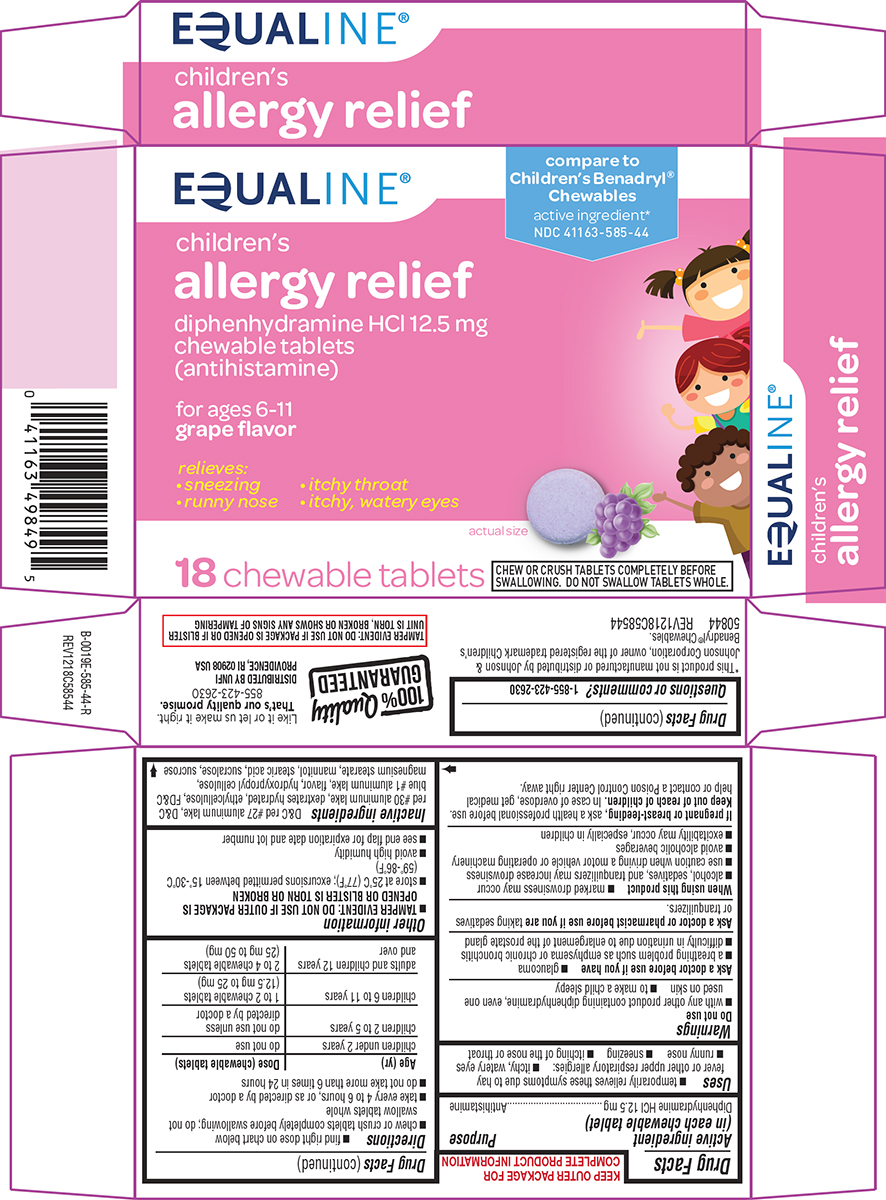
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
EQUALINE®
compare to
** Children’s Benadryl®**
** Chewables**
***active ingredient
NDC 41163-585-44
children's
allergy relief
diphenhydramine HCl 12.5 mg
chewable tablets
(antihistamine)
for ages 6-11****
** grape flavor**
relieves:
• sneezing • itchy throat
• runny nose • itchy, watery eyes
18 chewabletablets
actual size
CHEW OR CRUSH TABLETS COMPLETELY BEFORE SWALLOWING.
DO NOT SWALLOW TABLETS WHOLE.
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER
** UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING**
*This product is not manufactured or distributed by Johnson &
Johnson Corporation, owner of the registered trademark Children’s
Benadryl® Chewables.
50844 REV1218C58544
100% Quality
** GUARANTEED**
Like it or let us make it right.
That’s our quality promise.
855-423-2630
DISTRIBUTED BY UNFI
** PROVIDENCE, RI 02908 USA**
Equaline 44-585
INDICATIONS & USAGE SECTION
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- itchy, watery eyes
- runny nose
- sneezing
- itching of the nose or throat
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each chewable tablet)
Diphenhydramine HCl 12.5 mg
OTC - PURPOSE SECTION
Purpose
Antihistamine
WARNINGS SECTION
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- glaucoma
- a breathing problem such as emphysema or chronic bronchitis
- difficulty in urination due to enlargement of the prostate gland
Ask a doctor or pharmacist before use if you are
taking sedatives or tranquilizers.
When using this product
- marked drowsiness may occur
- alcohol, sedatives, and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- avoid alcoholic beverages
- excitability may occur, especially in children
If pregnant or breast-feeding,
ask a health professional before use.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- find right dose on chart below
- chew or crush tablets completely before swallowing; do not swallow tablets whole
- take every 4 to 6 hours, or as directed by a doctor
- do not take more than 6 times in 24 hours
|
Age (yr) |
Dose (chewable tablets) |
|
children under 2 years |
do not use |
|
children 2 to 5 years |
do not use unless directed by a doctor |
|
children 6 to 11 years |
1 to 2 chewable tablets (12.5 mg to 25 mg) |
|
adults and children 12 years and over |
2 to 4 chewable tablets (25 mg to 50 mg) |
STORAGE AND HANDLING SECTION
Other information
*TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN
- store at 25°C (77°F); excursions permitted between 15°-30°C (59°-86°F)
- avoid high humidity
- see end flap for expiration date and lot number
INACTIVE INGREDIENT SECTION
Inactive ingredients
D&C red #27 aluminum lake, D&C red #30 aluminum lake, dextrates hydrated, ethylcellulose, FD&C blue #1 aluminum lake, flavor, hydroxypropyl cellulose, magnesium stearate, mannitol, stearic acid, sucralose, sucrose
OTC - QUESTIONS SECTION
Questions or comments?
1-855-423-2630
