Pindolol
PINDOLOL TABLETS USP
611be921-e9b6-4800-80b2-c2d4f677cdb2
HUMAN PRESCRIPTION DRUG LABEL
Sep 19, 2025
ANI Pharmaceuticals, Inc.
DUNS: 145588013
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Pindolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Pindolol
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 62559-561-01
Pindolol Tablets USP, 10 mg
Rx only
100 Tablets
****
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Pindolol tablets are contraindicated in: 1) bronchial asthma; 2) overt cardiac failure; 3) cardiogenic shock; 4) second and third degree heart block; 5) severe bradycardia. (See WARNINGS.)
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Most adverse reactions have been mild. The incidences listed in the following table are derived from 12-week comparative double-blind, parallel design trials in hypertensive patients given pindolol as monotherapy, given various active control drugs as monotherapy, or given placebo. Data for pindolol and the positive controls were pooled from several trials because no striking differences were seen in the individual studies, with one exception. When considering all adverse reactions reported, the frequency of edema was noticeably higher in positive control trials (16% pindolol vs. 9% positive control) than in placebo controlled trials (6% pindolol vs. 3% placebo). The table includes adverse reactions either volunteered or elicited, and at least possibly drug-related, which were reported in greater than 2% of pindolol patients and other selected important reactions.
|
ADVERSE REACTIONS WHICH WERE VOLUNTEERED OR ELICITED | |||
|
(and at least possibly drug-related) | |||
|
Pindolol |
Active Controls* |
Placebo | |
|
Body System/ | |||
|
Central Nervous System | |||
|
5 |
0 |
6 |
|
9 |
11 |
1 |
|
8 |
4 |
4 |
|
<1 |
0 |
0 |
|
10 |
3 |
10 |
|
7 |
3 |
5 |
|
4 |
2 |
1 |
|
Autonomic Nervous System | |||
|
3 |
1 |
6 |
|
Cardiovascular | |||
|
5 |
4 |
6 |
|
6 |
3 |
1 |
|
<1 |
<1 |
0 |
|
<1 |
1 |
0 |
|
Musculoskeletal | |||
|
3 |
1 |
3 |
|
7 |
4 |
4 |
|
3 |
1 |
0 |
|
10 |
9 |
8 |
|
Gastrointestinal | |||
|
4 |
4 |
5 |
|
5 |
2 |
1 |
|
Skin | |||
|
1 |
<1 |
0 |
|
<1 |
<1 |
1 |
- Active Controls: Patients received either propranolol, α-methyldopa or a diuretic (hydrochlorothiazide or chlorthalidone).
The following selected (potentially important) adverse reactions were seen in 2% or fewer patients and their relationship to pindolol is uncertain. CENTRAL NERVOUS SYSTEM: anxiety, lethargy; AUTONOMIC NERVOUS SYSTEM: visual disturbances, hyperhidrosis; CARDIOVASCULAR: bradycardia, claudication, cold extremities, heart block, hypotension, syncope, tachycardia, weight gain; GASTROINTESTINAL: diarrhea, vomiting; RESPIRATORY: wheezing; UROGENITAL: impotence, pollakiuria; MISCELLANEOUS: eye discomfort or burning eyes.
POTENTIAL ADVERSE EFFECTS
In addition, other adverse effects not aforementioned have been reported with other beta-adrenergic blocking agents and should be considered potential adverse effects of pindolol.
Central Nervous System: Reversible mental depression progressing to catatonia; an acute reversible syndrome characterized by disorientation for time and place, short-term memory loss, emotional lability, slightly clouded sensorium, and decreased performance on neuropsychometrics.
Cardiovascular: Intensification of AV block. (See CONTRAINDICATIONS.)
Allergic: Erythematous rash; fever combined with aching and sore throat; laryngospasm; respiratory distress.
Hematologic: Agranulocytosis; thrombocytopenic and nonthrombocytopenic purpura.
Gastrointestinal: Mesenteric arterial thrombosis; ischemic colitis.
Miscellaneous: Reversible alopecia; Peyronie’s disease.
The oculomucocutaneous syndrome associated with the beta-blocker practolol has not been reported with pindolol during investigational use and extensive foreign experience amounting to over 4 million patient-years.
To report SUSPECTED ADVERSE REACTIONS, contact ANI Pharmaceuticals, Inc. at 1-855-204-1431 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
DESCRIPTION
Pindolol, a synthetic beta-adrenergic receptor blocking agent with intrinsic sympathomimetic activity is 1-(Indol-4-yloxy)-3-(isopropylamino)-2-propanol.
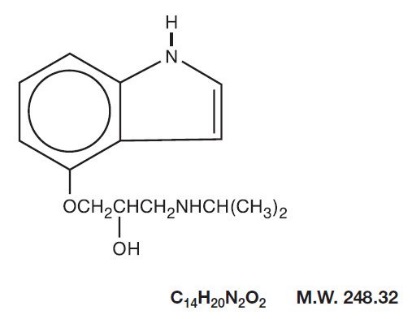
Pindolol, USP is a white to off-white, crystalline powder having a faint odor which is practically insoluble in water; slightly soluble in methanol; and very slightly soluble in chloroform.
Each tablet for oral administration contains pindolol and the following inactive ingredients: microcrystalline cellulose, pregelatinized starch, colloidal silicon dioxide and magnesium stearate.
HOW SUPPLIED SECTION
HOW SUPPLIED
Pindolol Tablets USP are available as 5 mg and 10 mg tablets.
The 5 mg tablets are a 1/4”, round, white, standard cup tablet, scored, debossedGG 438 on one side and plain on the reverse side. They are available in bottles of 100 tablets (NDC 62559-560-01).
The 10 mg tablets are a 5/16”, round, white, standard cup tablet, scored, debossedGG 439on one side and plain on the reverse side. They are available in bottles of 100 tablets (NDC 62559-561-01).
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature].
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
All brand names listed are the registered trademarks of their respective owners and are not trademarks of ANI Pharmaceuticals, Inc.
Distributed by:
ANI Pharmaceuticals, Inc.
Baudette, MN 56623

Issued: 04/2025
LB4535-01
