Nystatin
NYSTATIN ORAL SUSPENSION, USP
8d3531b9-13b4-471b-8315-9d05c80208f6
HUMAN PRESCRIPTION DRUG LABEL
Jul 31, 2025
ATLANTIC BIOLOGICALS CORP.
DUNS: 047437707
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Nystatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (24)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 60 mL Bottle Carton
NYSTATIN ORAL
SUSPENSION, USP
(100,000 units
per mL)
Fruit Flavored
Rx Only


ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Nystatin is well tolerated even with prolonged therapy. Oral irritation and sensitization have been reported. (SeePRECAUTIONS: General).
Gastrointestinal:Diarrhea (including one case of bloody diarrhea), nausea, vomiting, gastrointestinal upset/disturbances.
Dermatologic:Rash, including urticaria has been reported rarely. Stevens- Johnson syndrome has been reported very rarely.
Other:Tachycardia, bronchospasm, facial swelling, and non-specific myalgia have also been rarely reported.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Pharmacokinetics
Gastrointestinal absorption of nystatin is insignificant. Most orally administered nystatin is passed unchanged in the stool. In patients with renal insufficiency receiving oral therapy with conventional dosage forms, significant plasma concentrations of nystatin may occasionally occur.
Microbiology
Nystatin is both fungistatic and fungicidal in vitroagainst a wide variety of yeasts and yeast-like fungi. Candida albicansdemonstrates no significant resistance to nystatin in vitroon repeated subculture in increasing levels of nystatin; other Candidaspecies become quite resistant. Generally, resistance does not develop in vivo. Nystatin acts by binding to sterols in the cell membrane of susceptible Candidaspecies with a resultant change in membrane permeability allowing leakage of intracellular components. Nystatin exhibits no appreciable activity against bacteria, protozoa, or viruses.
SPL UNCLASSIFIED SECTION
Rx Only
Product No.: 8537
Manufactured For:
** Wockhardt USA, LLC**
** Parsippany, NJ 07054**
Manufactured By:
** Morton Grove Pharmaceuticals, Inc.**
** Morton Grove, IL 60053**
28537B
REV. 09-09
Distributed by Atlantic Biologicals
Miami, Fl 33179
DESCRIPTION SECTION
DESCRIPTION
Nystatin is an antimycotic polyene antibiotic obtained from Streptomyces noursei. Structural formula:
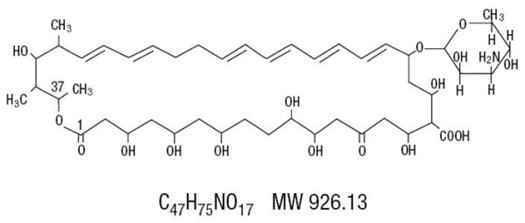
Nystatin Oral Suspension, for oral administration, contains 100,000 USP Nystatin Units per mL. Inactive ingredients: alcohol (≤ 1% v/v), artificial wild cherry flavor, banana flavor, D&C yellow #10, FD&C red #40, glycerin, USP, magnesium aluminum silicate, methylparaben, NF, potassium phosphate dibasic, USP, propylene glycol, USP, propylparaben, NF, purified water, USP and sucrose 33.5%. May also contain citric acid, USP for pH adjustment.
