Ledipasvir and Sofosbuvir
These highlights do not include all the information needed to use LEDIPASVIR and SOFOSBUVIR TABLETS safely and effectively. See full prescribing information for LEDIPASVIR and SOFOSBUVIR TABLETS. LEDIPASVIR and SOFOSBUVIR tablets, for oral use, 90 mg/400 mg (Authorized generic of HARVONI) Initial U.S. Approval: 2014
46f4a73b-0cd6-4902-9092-3ac79e882c1a
HUMAN PRESCRIPTION DRUG LABEL
Jun 25, 2021
Asegua Therapeutics LLC
DUNS: 116670954
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Ledipasvir and Sofosbuvir
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (15)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 14 Tablet Blister Pack Card Carton
NDC 72626-2601-1
ASEGUA™
THERAPEUTICS
Ledipasvir and Sofosbuvir
90 mg / 400 mg
tablets
Take 1 tablet once daily
Rx only
Note to pharmacist:
Do not cover ALERT box with pharmacy label.
ALERT: Find out about medicines that should
NOT be taken with Ledipasvir and Sofosbuvir
Contents:
Two blister cards
Each blister card contains 14 tablets.
Authorized generic of Harvoni®

BOXED WARNING SECTION
WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH
HCV AND HBV
See full prescribing information for complete boxed warning.
Hepatitis B virus (HBV) reactivation has been reported, in some cases resulting in fulminant hepatitis, hepatic failure, and death. (5.1)
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Ledipasvir and sofosbuvir is indicated for the treatment of adults and pediatric patients 3 years of age and older with chronic hepatitis C virus (HCV) [see Dosage and Administration (2.2 and 2.3) and Clinical Studies (14)]:
- genotype 1, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis
- genotype 1 infection with decompensated cirrhosis, for use in combination with ribavirin
- genotype 1 or 4 infection who are liver transplant recipients without cirrhosis or with compensated cirrhosis, for use in combination with ribavirin
Ledipasvir and sofosbuvir is a fixed-dose combination of ledipasvir, a hepatitis C virus (HCV) NS5A inhibitor, and sofosbuvir, an HCV nucleotide analog NS5B polymerase inhibitor, and is indicated for the treatment of chronic hepatitis C virus (HCV) in adults and pediatric patients 3 years of age and older:
- Genotype 1, 4, 5, or 6 infection without cirrhosis or with compensated cirrhosis
- Genotype 1 infection with decompensated cirrhosis, in combination with ribavirin
- Genotype 1 or 4 infection who are liver transplant recipients without cirrhosis or with compensated cirrhosis, in combination with ribavirin. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
If ledipasvir and sofosbuvir is administered with ribavirin, the contraindications to ribavirin also apply to this combination regimen. Refer to the ribavirin prescribing information for a list of contraindications for ribavirin [see Dosage and Administration (2.2)].
If used in combination with ribavirin, all contraindications to ribavirin also apply to ledipasvir and sofosbuvir combination therapy. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV
and HBV
Hepatitis B virus (HBV) reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct acting antivirals, and who were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Cases have been reported in patients who are HBsAg positive and also in patients with serologic evidence of resolved HBV infection (i.e., HBsAg negative and anti- HBc positive). HBV reactivation has also been reported in patients receiving certain immunosuppressants or chemotherapeutic agents; the risk of HBV reactivation associated with treatment with HCV direct-acting antivirals may be increased in these patients.
HBV reactivation is characterized as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level. In patients with resolved HBV infection, reappearance of HBsAg can occur. Reactivation of HBV replication may be accompanied by hepatitis, i.e., increases in aminotransferase levels and, in severe cases, increases in bilirubin levels, liver failure, and death can occur.
Test all patients for evidence of current or prior HBV infection by measuring HBsAg and anti-HBc before initiating HCV treatment with ledipasvir and sofosbuvir. In patients with serologic evidence of HBV infection, monitor for clinical and laboratory signs of hepatitis flare or HBV reactivation during HCV treatment with ledipasvir and sofosbuvir and during post-treatment follow- up. Initiate appropriate patient management for HBV infection as clinically indicated.
5.2 Serious Symptomatic Bradycardia When Coadministered with Amiodarone
Postmarketing cases of symptomatic bradycardia, as well as fatal cardiac arrest and cases requiring pacemaker intervention, have been reported when amiodarone is coadministered with ledipasvir and sofosbuvir. Bradycardia has generally occurred within hours to days, but cases have been observed up to 2 weeks after initiating HCV treatment. Patients also taking beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease, may be at increased risk for symptomatic bradycardia with coadministration of amiodarone. Bradycardia generally resolved after discontinuation of HCV treatment. The mechanism for this effect is unknown.
Coadministration of amiodarone with ledipasvir and sofosbuvir is not recommended. For patients taking amiodarone who have no other alternative, viable treatment options and who will be coadministered ledipasvir and sofosbuvir:
- Counsel patients about the risk of serious symptomatic bradycardia
- Cardiac monitoring in an in-patient setting for the first 48 hours of coadministration is recommended, after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.
Patients who are taking ledipasvir and sofosbuvir who need to start amiodarone therapy due to no other alternative, viable treatment options should undergo similar cardiac monitoring as outlined above.
Due to amiodarone's long half-life, patients discontinuing amiodarone just prior to starting ledipasvir and sofosbuvir should also undergo similar cardiac monitoring as outlined above.
Patients who develop signs or symptoms of bradycardia should seek medical evaluation immediately. Symptoms may include near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pains, confusion, or memory problems [see Adverse Reactions (6.2), Drug Interactions (7.2)].
5.3 Risk of Reduced Therapeutic Effect Due to Use with P-gp Inducers
The concomitant use of ledipasvir and sofosbuvir and P-gp inducers may significantly decrease ledipasvir and sofosbuvir plasma concentrations and may lead to a reduced therapeutic effect of ledipasvir and sofosbuvir. Therefore, the use of ledipasvir and sofosbuvir with P-gp inducers (e.g., rifampin, St. John's wort) is not recommended [see Drug Interactions (7.2)].
5.4 Risks Associated with Ribavirin Combination Treatment
If ledipasvir and sofosbuvir is administered with ribavirin, the warnings and precautions for ribavirin, in particular the pregnancy avoidance warning, apply to this combination regimen. Refer to the ribavirin prescribing information for a full list of the warnings and precautions for ribavirin [see Dosage and Administration (2.2)].
- Risk of Hepatitis B Virus Reactivation: Test all patients for evidence of current or prior HBV infection before initiation of HCV treatment. Monitor HCV/HBV coinfected patients for HBV reactivation and hepatitis flare during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated. (5.1)
- Bradycardia with amiodarone coadministration: Serious symptomatic bradycardia may occur in patients taking amiodarone, particularly in patients also receiving beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease. Coadministration of amiodarone with ledipasvir and sofosbuvir is not recommended. In patients without alternative, viable treatment options, cardiac monitoring is recommended. (5.2, 6.2, 7.2)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following serious adverse reactions are described below and elsewhere in labeling:
- Serious Symptomatic Bradycardia When Coadministered with Amiodarone [see Warnings and Precautions (5.2)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
If ledipasvir and sofosbuvir is administered with ribavirin to adults, refer to the prescribing information for ribavirin for a description of ribavirin- associated adverse reactions.
Clinical Trials in Adult Subjects
The safety assessment of ledipasvir and sofosbuvir was based on pooled data from three randomized, open-label Phase 3 clinical trials (ION-3, ION-1, and ION-2) of subjects with genotype 1 HCV with compensated liver disease (with and without cirrhosis) including 215, 539, and 326 subjects who received ledipasvir and sofosbuvir tablets (90 mg/400 mg) once daily by mouth for 8, 12, and 24 weeks, respectively [see Clinical Studies (14)].
The proportion of subjects who permanently discontinued treatment due to adverse events was 0%, less than 1%, and 1% for subjects receiving ledipasvir and sofosbuvir for 8, 12, and 24 weeks, respectively.
The most common adverse reactions (at least 10%) were fatigue and headache in subjects treated with 8, 12, or 24 weeks of ledipasvir and sofosbuvir.
Table 4 lists adverse reactions (adverse events assessed as causally related by the investigator, all grades) observed in at least 5% of subjects receiving 8, 12, or 24 weeks of treatment with ledipasvir and sofosbuvir in clinical trials. The majority of adverse reactions presented in Table 4 occurred at severity of grade 1. The side-by-side tabulation is to simplify presentation; direct comparison across trials should not be made due to differing trial designs.
Table 4 Adverse Reactions (All Grades) Reported in ≥5% of Subjects Receiving 8, 12, or 24 Weeks of Treatment with Ledipasvir and Sofosbuvir|
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir | |
|---|---|---|---|
|
Fatigue |
16% |
13% |
18% |
|
Headache |
11% |
14% |
17% |
|
Nausea |
6% |
7% |
9% |
|
Diarrhea |
4% |
3% |
7% |
|
Insomnia |
3% |
5% |
6% |
The safety assessment of ledipasvir and sofosbuvir was also based on pooled data from three open-label trials (Study 1119, ION-4, and ELECTRON-2) in 118 subjects with chronic HCV genotype 4, 5, or 6 infection with compensated liver disease (with or without cirrhosis) [see Clinical Studies (14.3)]. The subjects received ledipasvir and sofosbuvir tablets (90 mg/400 mg) once daily by mouth for 12 weeks. The safety profile in subjects with chronic HCV genotype 4, 5, or 6 infection with compensated liver disease was similar to that observed in subjects with chronic HCV genotype 1 infection with compensated liver disease. The most common adverse reactions occurring in at least 10% of subjects were asthenia (18%), headache (14%), and fatigue (10%).
Adverse Reactions in Subjects with Cirrhosis
The safety assessment of ledipasvir and sofosbuvir with or without ribavirin was based on a randomized, double-blind and placebo-controlled trial in treatment-experienced genotype 1 subjects with compensated cirrhosis and was compared to placebo in the SIRIUS trial. Subjects were randomized to receive 24 weeks of ledipasvir and sofosbuvir tablets (90 mg/400 mg) once daily by mouth without ribavirin or 12 weeks of placebo followed by 12 weeks of ledipasvir and sofosbuvir tablets (90 mg/400 mg) once daily by mouth + ribavirin [see Clinical Studies (14.2)]. Table 5 presents the adverse reactions, as defined above, that occurred with at least 5% greater frequency in subjects treated with 24 weeks of ledipasvir and sofosbuvir or 12 weeks of ledipasvir and sofosbuvir + ribavirin, compared to those reported for 12 weeks of placebo. The majority of the adverse reactions presented in Table 5 were Grade 1 or 2 in severity.
Table 5 Adverse Reactions with ≥5% Greater Frequency Reported in Treatment-Experienced Subjects with Cirrhosis Receiving Ledipasvir and Sofosbuvir for 24 Weeks or Ledipasvir and Sofosbuvir + Ribavirin for 12 Weeks Compared to Placebo for 12 weeks|
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir + RBV |
Placebo | |
|---|---|---|---|
|
RBV=ribavirin | |||
|
Asthenia |
31% |
36% |
23% |
|
Headache |
29% |
13% |
16% |
|
Fatigue |
18% |
4% |
1% |
|
Cough |
5% |
11% |
1% |
|
Myalgia |
9% |
4% |
0 |
|
Dyspnea |
3% |
9% |
1% |
|
Irritability |
8% |
7% |
1% |
|
Dizziness |
5% |
1% |
0 |
Adverse Reactions in Subjects Coinfected with HIV-1
The safety assessment of ledipasvir and sofosbuvir was based on an open-label clinical trial in 335 genotype 1 or 4 subjects with HCV/HIV-1 coinfection who were on stable antiretroviral therapy in Study ION-4 [see Clinical Studies (14.4)]. The safety profile in HCV/HIV-1 coinfected subjects was similar to that observed in HCV mono-infected subjects. The most common adverse reactions occurring in at least 10% of subjects were headache (20%) and fatigue (17%).
Adverse Reactions in Liver Transplant Recipients and/or Subjects with Decompensated Cirrhosis
The safety assessment of ledipasvir and sofosbuvir with ribavirin in liver transplant recipients and/or those who had decompensated liver disease was based on pooled data from two Phase 2 open-label clinical trials including 336 subjects who received ledipasvir and sofosbuvir tablets (90 mg/400 mg) plus ribavirin for 12 weeks. Subjects with Child-Pugh-Turcotte (CPT) scores greater than 12 were excluded from the trials [see Clinical Studies (14.5)].
The adverse events observed were consistent with the expected clinical sequelae of liver transplantation and/or decompensated liver disease, or the known safety profile of ledipasvir and sofosbuvir and/or ribavirin.
Decreases in hemoglobin to less than 10 g/dL and 8.5 g/dL during treatment were observed in 38% and 13% of subjects treated with ledipasvir and sofosbuvir plus ribavirin for 12 weeks, respectively. Ribavirin was permanently discontinued in 11% of subjects treated with ledipasvir and sofosbuvir plus ribavirin for 12 weeks.
Liver Transplant Recipients with Compensated Liver Disease:
Among the 174 liver transplant recipients with compensated liver disease who received ledipasvir and sofosbuvir tablets (90 mg/400 mg) with ribavirin for 12 weeks, 2 (1%) subjects permanently discontinued ledipasvir and sofosbuvir due to an adverse event.
Subjects with Decompensated Liver Disease:
Among the 162 subjects with decompensated liver disease (pre- or post- transplant) who received ledipasvir and sofosbuvir tablets (90 mg/400 mg) with ribavirin for 12 weeks, 7 (4%) subjects died, 4 (2%) subjects underwent liver transplantation, and 1 subject (<1%) underwent liver transplantation and died during treatment or within 30 days after discontinuation of treatment. Because these events occurred in patients with advanced liver disease who are at risk of progression of liver disease including liver failure and death, it is not possible to reliably assess the contribution of drug effect to outcomes. A total of 4 (2%) subjects permanently discontinued ledipasvir and sofosbuvir due to an adverse event.
Less Common Adverse Reactions Reported in Clinical Trials (less than 5%): The following adverse reactions occurred in less than 5% of subjects receiving ledipasvir and sofosbuvir tablets (90 mg/400 mg) in any one trial. These events have been included because of their seriousness or assessment of potential causal relationship.
Psychiatric disorders: depression (including in subjects with pre-existing history of psychiatric illness).
Depression (particularly in subjects with pre-existing history of psychiatric illness) occurred in subjects receiving sofosbuvir containing regimens. Suicidal ideation and suicide have occurred in less than 1% of subjects treated with sofosbuvir in combination with ribavirin or pegylated interferon/ribavirin in other clinical trials.
Laboratory Abnormalities
Bilirubin Elevations: Bilirubin elevations of greater than 1.5×ULN were observed in 3%, less than 1%, and 2% of subjects treated with ledipasvir and sofosbuvir tablets (90 mg/400 mg) for 8, 12, and 24 weeks, respectively. Bilirubin elevations of greater than 1.5×ULN were observed in 3%, 11%, and 3% of subjects with compensated cirrhosis treated with placebo, ledipasvir and sofosbuvir + ribavirin for 12 weeks, and ledipasvir and sofosbuvir for 24 weeks, respectively, in the SIRIUS trial.
Lipase Elevations: Transient, asymptomatic lipase elevations of greater than 3×ULN were observed in less than 1%, 2%, and 3% of subjects treated with ledipasvir and sofosbuvir tablets (90 mg/400 mg) for 8, 12, and 24 weeks, respectively. Transient, asymptomatic lipase elevations of greater than 3×ULN were observed in 1%, 3%, and 9% of subjects with compensated cirrhosis treated with placebo, ledipasvir and sofosbuvir + ribavirin for 12 weeks, and ledipasvir and sofosbuvir for 24 weeks, respectively, in the SIRIUS trial.
Creatine Kinase: Creatine kinase was not assessed in Phase 3 trials ION-3, ION-1, or ION-2 of ledipasvir and sofosbuvir. Creatine kinase was assessed in the ION-4 trial. Isolated, asymptomatic creatine kinase elevations of greater than or equal to 10×ULN was observed in 1% of subjects treated with ledipasvir and sofosbuvir tablets (90 mg/400 mg) for 12 weeks in the ION-4 trial and has also been previously reported in subjects treated with sofosbuvir in combination with ribavirin or peginterferon/ribavirin in other clinical trials.
Adverse Reactions in Adults with Severe Renal Impairment, Including those on Dialysis
In an open-label trial (Trial 0154) in which adults with HCV with compensated liver disease (with or without cirrhosis) and severe renal impairment received ledipasvir and sofosbuvir tablets (90 mg/400 mg) for 12 weeks (N=18), the most common adverse reaction was fatigue (17%) [see Clinical Studies (14.6)].
In an open-label clinical trial, Trial 4063, a total of 95 adults with HCV with compensated liver disease (with or without cirrhosis) and ESRD requiring dialysis received ledipasvir and sofosbuvir tablets (90 mg/400 mg) for 8 (n=45), 12 (n=31), or 24 (n=19) weeks. The most common adverse reactions were insomnia and headache (each reported in 4% of subjects overall) [see Clinical Studies (14.6)].
Adverse Reactions in Pediatric Subjects 3 Years of Age and Older
The safety assessment of ledipasvir and sofosbuvir tablets (90 mg/400 mg), ledipasvir and sofosbuvir (HARVONI) tablets (45 mg/200 mg), or ledipasvir and sofosbuvir (HARVONI) oral pellets in pediatric subjects 3 years of age and older is based on data from a Phase 2, open-label clinical trial (Study 1116). In total, 226 subjects were enrolled, which included 223 subjects without cirrhosis or with compensated cirrhosis who were treated with ledipasvir and sofosbuvir for 12 weeks; one genotype 1 treatment-experienced subject with cirrhosis who was treated with ledipasvir and sofosbuvir for 24 weeks; and two genotype 3 subjects who were treated with ledipasvir and sofosbuvir + ribavirin for 24 weeks. The adverse reactions observed were consistent with those observed in clinical studies of ledipasvir and sofosbuvir tablets (90 mg/400 mg) in adults. Limited safety data are available in pediatric subjects receiving ledipasvir and sofosbuvir for 24 weeks. No Grade 3 or 4 adverse reactions or discontinuation due to an adverse reaction was observed in those pediatric subjects receiving ledipasvir and sofosbuvir for 24 weeks [see Clinical Studies (14.7)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ledipasvir and sofosbuvir. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac Disorders
Serious symptomatic bradycardia has been reported in patients taking amiodarone who initiate treatment with ledipasvir and sofosbuvir [see Warnings and Precautions (5.2), Drug Interactions (7.2)].
Skin and Subcutaneous Tissue Disorders
Skin rashes, sometimes with blisters or angioedema-like swelling
Angioedema
- The most common adverse reactions (incidence greater than or equal to 10%, all grades) observed with treatment with ledipasvir and sofosbuvir were fatigue, headache, and asthenia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Asegua Therapeutics at 1-800-445-3235 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Potential for Drug Interaction
Any interactions that have been identified with ledipasvir or sofosbuvir individually may occur with ledipasvir and sofosbuvir.
After oral administration of ledipasvir and sofosbuvir, sofosbuvir is rapidly absorbed and subject to extensive first-pass hepatic extraction. In clinical pharmacology studies, both sofosbuvir and the inactive metabolite GS-331007 were monitored for purposes of pharmacokinetic analyses.
Ledipasvir is an inhibitor of the drug transporters P-gp and breast cancer resistance protein (BCRP) and may increase intestinal absorption of coadministered substrates for these transporters.
Ledipasvir and sofosbuvir are substrates of drug transporters P-gp and BCRP while GS-331007 is not. P-gp inducers (e.g., rifampin, St. John's wort) may decrease ledipasvir and sofosbuvir plasma concentrations, leading to reduced therapeutic effect of ledipasvir and sofosbuvir, and the use with P-gp inducers is not recommended with ledipasvir and sofosbuvir [see Warnings and Precautions (5.3)].
7.2 Established and Potentially Significant Drug Interactions
Clearance of HCV infection with direct acting antivirals may lead to changes in hepatic function, which may impact the safe and effective use of concomitant medications. For example, altered blood glucose control resulting in serious symptomatic hypoglycemia has been reported in diabetic patients in postmarketing case reports and published epidemiological studies. Management of hypoglycemia in these cases required either discontinuation or dose modification of concomitant medications used for diabetes treatment.
Frequent monitoring of relevant laboratory parameters (e.g., International Normalized Ratio [INR] in patients taking warfarin, blood glucose levels in diabetic patients) or drug concentrations of concomitant medications such as cytochrome P450 substrates with a narrow therapeutic index (e.g., certain immunosuppressants) is recommended to ensure safe and effective use. Dose adjustments of concomitant medications may be necessary.
Table 6 provides a listing of established or potentially clinically significant drug interactions. The drug interactions described are based on studies conducted with either ledipasvir and sofosbuvir tablets (90 mg/400 mg) or ledipasvir and sofosbuvir as individual agents, or are predicted drug interactions that may occur with ledipasvir and sofosbuvir [see Warnings and Precautions (5.2, 5.3) and Clinical Pharmacology (12.3)].
Table 6 Potentially Significant Drug Interactions: Alteration in Dose or Regimen May Be Recommended Based on Drug Interaction Studies or Predicted Interaction*|
Concomitant Drug Class: Drug Name |
Effect on Concentration† |
Clinical Comment |
|---|---|---|
|
tenofovir DF = tenofovir disoproxil fumarate | ||
| ||
|
Acid Reducing Agents: |
↓ ledipasvir |
Ledipasvir solubility decreases as pH increases. Drugs that increase gastric pH are expected to decrease concentration of ledipasvir. |
|
Antacids (e.g., aluminum and magnesium hydroxide) |
It is recommended to separate antacid and ledipasvir and sofosbuvir administration by 4 hours. | |
|
H2-receptor antagonists‡ (e.g., famotidine) |
H2-receptor antagonists may be administered simultaneously with or 12 hours apart from ledipasvir and sofosbuvir at a dose that does not exceed doses comparable to famotidine 40 mg twice daily. | |
|
Proton-pump inhibitors‡ (e.g., omeprazole) |
Proton-pump inhibitor doses comparable to omeprazole 20 mg or lower can be administered simultaneously with ledipasvir and sofosbuvir under fasted conditions. | |
|
Antiarrhythmics: |
Effect on amiodarone, ledipasvir, and sofosbuvir concentrations unknown |
Coadministration of amiodarone with ledipasvir and sofosbuvir may result in serious symptomatic bradycardia. The mechanism of this effect is unknown. Coadministration of amiodarone with ledipasvir and sofosbuvir is not recommended; if coadministration is required, cardiac monitoring is recommended [see Warnings and Precautions (5.2), Adverse Reactions (6.2)]. |
|
digoxin |
↑ digoxin |
Coadministration of ledipasvir and sofosbuvir with digoxin may increase the concentration of digoxin. Therapeutic concentration monitoring of digoxin is recommended when coadministered with ledipasvir and sofosbuvir. |
|
Anticonvulsants: |
↓ ledipasvir |
Coadministration of ledipasvir and sofosbuvir with carbamazepine, phenytoin, or phenobarbital is expected to decrease the concentration of ledipasvir and sofosbuvir, leading to reduced therapeutic effect of ledipasvir and sofosbuvir. Coadministration is not recommended. |
|
Antimycobacterials: |
↓ ledipasvir |
Coadministration of ledipasvir and sofosbuvir with rifampin, rifabutin, or rifapentine is not recommended [see Warnings and Precautions (5.3)]. |
|
HIV Antiretrovirals: | ||
|
Regimens containing tenofovir DF without an HIV protease inhibitor/ritonavir or cobicistat |
↑ tenofovir |
Monitor for tenofovir-associated adverse reactions in patients receiving ledipasvir and sofosbuvir concomitantly with a regimen containing tenofovir DF without an HIV protease inhibitor/ritonavir or cobicistat. Refer to Viread® or Truvada® prescribing information for recommendations on renal monitoring. |
|
Regimens containing tenofovir DF and an HIV protease inhibitor/ritonavir or cobicistat
|
↑ tenofovir |
The safety of increased tenofovir concentrations in the setting of ledipasvir
and sofosbuvir and an HIV protease inhibitor/ritonavir or cobicistat has not
been established. |
|
elvitegravir, cobicistat, emtricitabine, tenofovir DF |
↑ tenofovir |
The safety of increased tenofovir concentrations in the setting of ledipasvir and sofosbuvir and the combination of elvitegravir, cobicistat, emtricitabine, and tenofovir DF has not been established. Coadministration is not recommended. |
|
tipranavir/ritonavir |
↓ ledipasvir |
Coadministration of ledipasvir and sofosbuvir with tipranavir/ritonavir is expected to decrease the concentration of ledipasvir and sofosbuvir, leading to reduced therapeutic effect of ledipasvir and sofosbuvir. Coadministration is not recommended. |
|
HCV Products: |
↑ ledipasvir |
Concentrations of ledipasvir and simeprevir are increased when simeprevir is coadministered with ledipasvir. Coadministration of ledipasvir and sofosbuvir with simeprevir is not recommended. |
|
Herbal Supplements: |
↓ ledipasvir |
Coadministration of ledipasvir and sofosbuvir with St. John's wort, a P-gp inducer, is not recommended [see Warnings and Precautions (5.3)]. |
|
HMG-CoA Reductase Inhibitors: |
↑ rosuvastatin |
Coadministration of ledipasvir and sofosbuvir with rosuvastatin may significantly increase the concentration of rosuvastatin, which is associated with increased risk of myopathy, including rhabdomyolysis. Coadministration of ledipasvir and sofosbuvir with rosuvastatin is not recommended. |
|
atorvastatin |
↑ atorvastatin |
Coadministration of ledipasvir and sofosbuvir with atorvastatin may be associated with increased risk of myopathy, including rhabdomyolysis. Monitor closely for HMG-CoA reductase inhibitor-associated adverse reactions, such as myopathy and rhabdomyolysis. |
7.3 Drugs without Clinically Significant Interactions with Ledipasvir and
Sofosbuvir
Based on drug interaction studies conducted with ledipasvir, sofosbuvir, or ledipasvir and sofosbuvir tablets (90 mg/400 mg) no clinically significant drug interactions have been either observed or are expected when ledipasvir and sofosbuvir is used with the following drugs [see Clinical Pharmacology (12.3)]: abacavir, atazanavir/ritonavir, cyclosporine, darunavir/ritonavir, dolutegravir, efavirenz, elvitegravir/cobicistat/emtricitabine/tenofovir alafenamide, emtricitabine, lamivudine, methadone, midazolam, oral contraceptives, pravastatin, raltegravir, rilpivirine, tacrolimus, or verapamil. See Table 6 for use of ledipasvir and sofosbuvir with certain HIV antiretroviral regimens [see Drug Interactions (7.2)].
- Coadministration with amiodarone may result in serious symptomatic bradycardia. Use of ledipasvir and sofosbuvir with amiodarone is not recommended. (5.2, 6.2, 7.2)
- P-gp inducers (e.g., rifampin, St. John's wort): May alter concentrations of ledipasvir and sofosbuvir. Use of ledipasvir and sofosbuvir with P-gp inducers is not recommended. (5.3, 7, 12.3)
- Consult the full prescribing information prior to use for potential drug interactions. (5.2, 5.3, 7, 12.3)
- Clearance of HCV infection with direct acting antivirals may lead to changes in hepatic function, which may impact safe and effective use of concomitant medications. Frequent monitoring of relevant laboratory parameters (INR or blood glucose) and dose adjustments of certain concomitant medications may be necessary. (7.2)
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
|
Indications and Usage (1) |
8/2019 |
|
Dosage and Administration | |
|
Recommended Treatment Regimen and Duration in Patients 3 Years of Age and Older with Genotype 1, 4, 5, or 6 HCV (2.2) |
8/2019 |
|
Recommended Dosage in Pediatric Patients 3 Years of Age and Older (2.4) |
8/2019 |
|
Preparation and Administration of Ledipasvir and Sofosbuvir (HARVONI) Oral Pellets (2.5) |
8/2019 |
|
Renal Impairment (2.6) |
11/2019 |
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Ledipasvir and sofosbuvir tablets (90 mg/400 mg) are available as orange, diamond-shaped, film-coated tablets debossed with "ASE" on one side and "9875" on the other side of the tablet. Each tablet contains 90 mg ledipasvir and 400 mg sofosbuvir.
Tablets: 90 mg of ledipasvir and 400 mg of sofosbuvir. (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
If ledipasvir and sofosbuvir is administered with ribavirin, the combination regimen is contraindicated in pregnant women and in men whose female partners are pregnant. Refer to the ribavirin prescribing information for more information on ribavirin-associated risks of use during pregnancy.
No adequate human data are available to establish whether or not ledipasvir and sofosbuvir pose a risk to pregnancy outcomes. In animal reproduction studies, no evidence of adverse developmental outcomes was observed with ledipasvir or sofosbuvir at exposures greater than those in humans at the recommended human dose (RHD) [see Data]. During organogenesis in the rat and rabbit, systemic exposures (AUC) to ledipasvir were approximately 4 (rats) and 2 (rabbits) times the exposure in humans at the RHD, while exposures to the predominant circulating metabolite of sofosbuvir (GS-331007) were ≥3 (rats) and 7 (rabbits) times the exposure in humans at the RHD. In rat pre/postnatal development studies, maternal systemic exposures (AUC) to ledipasvir and GS-331007 were approximately 5 and 7 times, respectively, the exposure in humans at the RHD.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Data
Animal Data
Ledipasvir: Ledipasvir was administered orally to pregnant rats (up to 100 mg/kg/day) and rabbits (up to 180 mg/kg/day) on gestation days 6 to 18 and 7 to 20, respectively, and also to rats (oral doses up to 100 mg/kg/day) on gestation day 6 to lactation/post-partum day 20. No significant effects on embryo-fetal (rats and rabbits) or pre/postnatal (rats) development were observed at the highest doses tested. Systemic exposures (AUC) to ledipasvir were ≥4 (rats) and 2 (rabbits) times the exposure in humans at the RHD.
Sofosbuvir: Sofosbuvir was administered orally to pregnant rats (up to 500 mg/kg/day) and rabbits (up to 300 mg/kg/day) on gestation days 6 to 18 and 6 to 19, respectively, and also to rats (oral doses up to 500 mg/kg/day) on gestation day 6 to lactation/post-partum day 20. No significant effects on embryo-fetal (rats and rabbits) or pre/postnatal (rats) development were observed at the highest doses tested. Systemic exposures (AUC) to the predominant circulating metabolite of sofosbuvir (GS-331007) were ≥3 (rats) and 7 (rabbits) times the exposure in humans at the RHD, with exposures increasing during gestation from approximately 3 to 6 (rats) and 7 to 17 (rabbits) times the exposure in humans at the RHD.
8.2 Lactation
Risk Summary
It is not known whether ledipasvir, sofosbuvir, or their metabolites are present in human breast milk, affect human milk production or have effects on the breastfed infant. When administered to lactating rats, ledipasvir was detected in the plasma of nursing pups likely due to the presence of ledipasvir in milk, without clear effects on nursing pups [see Data]. The predominant circulating metabolite of sofosbuvir (GS-331007) was the primary component observed in the milk of lactating rats, without effect on nursing pups.
The development and health benefits of breastfeeding should be considered along with the mother's clinical need for ledipasvir and sofosbuvir and any potential adverse effects on the breastfed child from ledipasvir and sofosbuvir or from the underlying maternal condition.
If ledipasvir and sofosbuvir is administered with ribavirin, the nursing mother's information for ribavirin also applies to this combination regimen. Refer to the ribavirin prescribing information for more information on use during lactation.
Data
Ledipasvir: No effects of ledipasvir on growth and postnatal development were observed in nursing pups at the highest dose tested in rats. Maternal systemic exposure (AUC) to ledipasvir was approximately 5 times the exposure in humans at the RHD. Although not measured directly, ledipasvir was likely present in the milk of lactating rats, since systemic exposure (AUC) to ledipasvir of approximately 25% that of maternal exposure was observed in nursing pups on lactation day 10.
Sofosbuvir: No effects of sofosbuvir on growth and postnatal development were observed in nursing pups at the highest dose tested in rats. Maternal systemic exposure (AUC) to the predominant circulating metabolite of sofosbuvir (GS-331007) was approximately 7 times the exposure in humans at the RHD, with exposure of approximately 2% that of maternal exposure observed in nursing pups on lactation day 10. In a lactation study, sofosbuvir metabolites (primarily GS-331007) were excreted into the milk of lactating rats following administration of a single oral dose of sofosbuvir (20 mg/kg) on lactation day 2, with milk concentrations of approximately 10% that of maternal plasma concentrations observed 1 hour post-dose.
8.3 Females and Males of Reproductive Potential
If ledipasvir and sofosbuvir is administered with ribavirin, the information for ribavirin with regard to pregnancy testing, contraception, and infertility also applies to this combination regimen. Refer to ribavirin prescribing information for additional information.
8.4 Pediatric Use
The safety, pharmacokinetics, and efficacy of ledipasvir and sofosbuvir for treatment of HCV genotype 1 and 4 infection in treatment-naïve and treatment- experienced pediatric patients 3 years of age and older without cirrhosis or with compensated cirrhosis have been established in an open-label, multicenter clinical trial (Study 1116, N=226; 186 treatment-naïve, 40 treatment- experienced) and are comparable to that observed in adults.
The safety and efficacy of ledipasvir and sofosbuvir for treatment of HCV genotypes 5 or 6 infection in pediatric patients 3 years of age and older are supported by comparable ledipasvir, sofosbuvir, and GS-331007 exposures between adults and pediatric patients [see Dosage and Administration (2.2 and 2.4), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.3, 14.6, 14.7)]. Similar rationale is used to support dosing recommendations for pediatric patients with HCV genotype 1 infection who have decompensated cirrhosis (Child-Pugh B or C) and for pediatric patients with HCV genotype 1 and 4 infection who are liver transplant recipients without cirrhosis or with compensated cirrhosis.
In patients with severe renal impairment, including those requiring dialysis, exposures of GS-331007, the inactive metabolite of sofosbuvir, are increased [see Clinical Pharmacology (12.3)]. No data are available regarding the safety of ledipasvir and sofosbuvir in pediatric patients with renal impairment [see Use in Specific Populations (8.6)].
The safety and efficacy of ledipasvir and sofosbuvir have not been established in pediatric patients less than 3 years of age.
8.5 Geriatric Use
Clinical trials of ledipasvir and sofosbuvir included 225 subjects aged 65 and over (9% of total number of subjects in the clinical studies). No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out. No dosage adjustment of ledipasvir and sofosbuvir is warranted in geriatric patients [see Clinical Pharmacology (12.3)].
8.6 Renal Impairment
No dosage adjustment of ledipasvir and sofosbuvir is recommended for patients with mild, moderate, or severe renal impairment, including ESRD requiring dialysis [see Dosage and Administration (2.4), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.6)]. No safety data are available in subjects with both decompensated cirrhosis and severe renal impairment, including those on dialysis. Additionally, no safety data are available in pediatric patients with renal impairment [see Use in Specific Populations (8.4)]. Refer to ribavirin tablet prescribing information regarding use in patients with renal impairment.
8.7 Hepatic Impairment
No dosage adjustment of ledipasvir and sofosbuvir is recommended for patients with mild, moderate, or severe hepatic impairment (Child-Pugh Class A, B, or C) [see Dosage and Administration (2.3), Clinical Pharmacology (12.3) and Clinical Studies (14.5)].
Clinical and hepatic laboratory monitoring, as clinically indicated, is recommended for patients with decompensated cirrhosis receiving treatment with ledipasvir and sofosbuvir and ribavirin [see Adverse Reactions (6.1)].
- Pediatric Use: No data are available regarding the safety of ledipasvir and sofosbuvir in pediatric patients with renal impairment. (8.4)
OVERDOSAGE SECTION
10 OVERDOSAGE
No specific antidote is available for overdose with ledipasvir and sofosbuvir. If overdose occurs, the patient must be monitored for evidence of toxicity. Treatment of overdose with ledipasvir and sofosbuvir consists of general supportive measures including monitoring of vital signs as well as observation of the clinical status of the patient. Hemodialysis is unlikely to result in significant removal of ledipasvir since ledipasvir is highly bound to plasma protein. Hemodialysis can efficiently remove the predominant circulating metabolite of sofosbuvir, GS-331007, with an extraction ratio of 53%.
DESCRIPTION SECTION
11 DESCRIPTION
Ledipasvir and sofosbuvir tablets (90 mg/400 mg) are fixed-dose combination tablets containing ledipasvir and sofosbuvir for oral administration. Ledipasvir is an HCV NS5A inhibitor and sofosbuvir is a nucleotide analog inhibitor of HCV NS5B polymerase.
Each tablet contains 90 mg ledipasvir and 400 mg sofosbuvir. The tablets include the following inactive ingredients: colloidal silicon dioxide, copovidone, croscarmellose sodium, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film-coated with a coating material containing the following inactive ingredients: FD&C yellow #6/sunset yellow FCF aluminum lake, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide.
Ledipasvir: The IUPAC name for ledipasvir is methyl [(2S)-1-{(6S)-6-[5-(9,9-difluoro-7-{2-[(1R,3S,4S)-2-{(2S)-2-[(methoxycarbonyl)amino]-3-methylbutanoyl}-2-azabicyclo[2.2.1]hept-3-yl]-1H-benzimidazol-6-yl}-9H-fluoren-2-yl)-1H-imidazol-2-yl]-5-azaspiro[2.4]hept-5-yl}-3-methyl-1-oxobutan-2-yl]carbamate.
It has a molecular formula of C49H54F2N8O6 and a molecular weight of 889.00. It has the following structural formula:
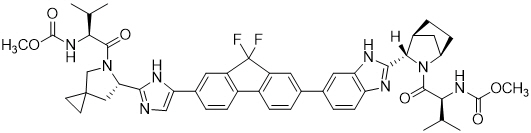
Ledipasvir is practically insoluble (less than 0.1 mg/mL) across the pH range of 3.0–7.5 and is slightly soluble below pH 2.3 (1.1 mg/mL).
Sofosbuvir: The IUPAC name for sofosbuvir is (S)-isopropyl 2-((S)-(((2R,3R,4R,5R)-5-(2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl)-4-fluoro-3-hydroxy-4-methyltetrahydrofuran-2-yl)methoxy)-(phenoxy)phosphorylamino)propanoate. It has a molecular formula of C22H29FN3O9P and a molecular weight of 529.45. It has the following structural formula:
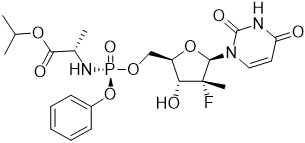
Sofosbuvir is a white to off-white crystalline solid with a solubility of at least 2 mg/mL across the pH range of 2–7.7 at 37°C and is slightly soluble in water.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis and Mutagenesis
Ledipasvir: Ledipasvir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes, and in vivo rat micronucleus assays.
Ledipasvir was not carcinogenic in a 6-month rasH2 transgenic mouse study (up to 300 mg/kg/day). Similarly, ledipasvir was not carcinogenic in a 2-year rat study (up to 100 mg/kg/day in males and 30 mg/kg/day in females), resulting in exposures approximately 10 and 4 times, respectively, higher than the exposure in humans at the recommended human dose (RHD).
Sofosbuvir: Sofosbuvir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosome aberration using human peripheral blood lymphocytes, and in vivo mouse micronucleus assays.
Sofosbuvir was not carcinogenic in a 2-year mouse study (up to 200 mg/kg/day in males and 600 mg/kg/day in females) and in a 2-year rat study (up to 750 mg/kg/day), resulting in exposures of the predominant circulating metabolite GS-331007 of approximately 4 and 18 times (in mice) and 8 and 10 times (in rats), in males and females respectively, the exposure in humans at the RHD.
Impairment of Fertility
Ledipasvir: Ledipasvir had no adverse effects on mating and fertility. In female rats, the mean number of corpora lutea and implantation sites were reduced slightly at maternal exposures approximately 3 times the exposure in humans at the RHD. At the highest dose levels without effects, exposures of ledipasvir were approximately 5 and 2 times, in males and females, respectively, the exposure in humans at the RHD.
Sofosbuvir: Sofosbuvir had no effects on embryo-fetal viability or on fertility when evaluated in rats. At the highest dose tested, exposure to the predominant circulating metabolite GS-331007 was approximately 5 times the exposure in humans at the RHD.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Description of Clinical Trials
The efficacy and safety of ledipasvir and sofosbuvir were evaluated in four trials in genotype 1 HCV mono-infected subjects including one trial exclusively in treatment-experienced subjects with compensated cirrhosis (Child-Pugh A); one trial in genotype 1 or 4 HCV/HIV-1 coinfected subjects; two trials in genotype 4, 5, or 6 HCV mono-infected subjects; two trials in genotype 1 or 4 HCV infected pretransplant subjects with decompensated cirrhosis (Child-Pugh B and C) or post-transplant with Metavir F0–F3 fibrosis, compensated cirrhosis, decompensated cirrhosis, or fibrosing cholestatic hepatitis (FCH); two trials in subjects with severe renal impairment (one of which included subjects requiring dialysis); and one trial in genotype 1 or 4 HCV pediatric subjects 3 years of age and older without cirrhosis or with compensated cirrhosis, as summarized in Table 10 [see Clinical Studies (14.2, 14.3, 14.4, 14.5, 14.6, and 14.7)]:
Table 10 Trials Conducted with Ledipasvir and Sofosbuvir with or without Ribavirin in Subjects with Chronic HCV Genotype 1, 4, 5, or 6 Infection|
Trial |
Population |
Study Arms (Number of Subjects Treated) |
|---|---|---|
|
ESRD = End stage renal disease; RBV = ribavirin; RI = Renal impairment; TN = Treatment-naïve subjects | ||
| ||
|
ION-3 * |
GT1, TN without cirrhosis |
Ledipasvir and sofosbuvir 8 weeks (215) |
|
ION-1 * |
GT1, TN with or without cirrhosis |
Ledipasvir and sofosbuvir 12 weeks (214) |
|
ION-2 * |
GT1, TE† with or without cirrhosis |
Ledipasvir and sofosbuvir 12 weeks (109) |
|
SIRIUS ‡ |
GT1, TE† with cirrhosis |
Ledipasvir and sofosbuvir + RBV 12 Weeks (77) |
|
ION-4 * |
GT1 and GT4 HCV/HIV-1 coinfected |
Ledipasvir and sofosbuvir 12 Weeks |
|
1119 * |
GT4 and GT5, TN and TE† with or without cirrhosis |
Ledipasvir and sofosbuvir 12 Weeks |
|
ELECTRON-2 * |
GT6, TN and TE† with or without cirrhosis |
Ledipasvir and sofosbuvir 12 Weeks (25) |
|
SOLAR-1 * and SOLAR-2 * |
GT1 and GT4 pre-transplant with decompensated cirrhosis or post-transplant with Metavir F0–F3 fibrosis, compensated cirrhosis, decompensated cirrhosis, or FCH |
Ledipasvir and sofosbuvir + RBV 12 Weeks (336) |
|
1116 * |
GT1 or 4 TN and TE with or without cirrhosis in pediatric subjects 3 years of age and older |
Ledipasvir and sofosbuvir 12 Weeks (223) |
|
0154 * |
GT1 TN and TE† with severe RI without dialysis |
Ledipasvir and sofosbuvir 12 weeks (18) |
|
4063 * |
GT1, 5, or 6 TN and TE§ with or without compensated cirrhosis, with ESRD requiring dialysis |
Ledipasvir and sofosbuvir 8 Weeks (45) |
Ledipasvir and sofosbuvir was administered once daily by mouth in these trials. For subjects without cirrhosis or with compensated cirrhosis who received ribavirin, the ribavirin dosage was 1000 mg per day for subjects weighing less than 75 kg or 1200 mg per day for subjects weighing at least 75 kg. For subjects with decompensated cirrhosis in SOLAR-1 and SOLAR-2 studies, the starting ribavirin dosage was 600 mg per day regardless of transplantation status. Ribavirin dose adjustments were performed according to the ribavirin labeling.
Serum HCV RNA values were measured during the clinical trials using the COBAS TaqMan HCV test (version 2.0), for use with the High Pure System in ION-3, ION-1, ION-2, SIRIUS, and ION-4 studies or the COBAS AmpliPrep/COBAS Taqman HCV test (version 2.0) in ELECTRON-2, 1119, SOLAR-1, SOLAR-2, and 1116 studies. The COBAS TaqMan HCV test (version 2.0) for use with the High Pure System has a lower limit of quantification (LLOQ) of 25 IU per mL and the COBAS AmpliPrep/COBAS Taqman HCV test (version 2.0) has a LLOQ of 15 IU per mL. Sustained virologic response (SVR12), defined as HCV RNA less than LLOQ at 12 weeks after the cessation of treatment, was the primary endpoint in studies in adults and the key efficacy endpoint in the study in pediatric subjects 12 years of age and older. Relapse was a secondary endpoint, which was defined as HCV RNA greater than or equal to LLOQ with 2 consecutive values or last available post-treatment measurement during the post-treatment period after achieving HCV RNA less than LLOQ at end of treatment.
14.2 Clinical Trials in Subjects with Genotype 1 HCV
Treatment-Naïve Adults without Cirrhosis ─ ION-3 (Study 0108)
ION-3 was a randomized, open-label trial in treatment-naïve non-cirrhotic subjects with genotype 1 HCV. Subjects were randomized in a 1:1:1 ratio to one of the following three treatment groups and stratified by HCV genotype (1a vs 1b): ledipasvir and sofosbuvir tablets (90 mg/400 mg) for 8 weeks, ledipasvir and sofosbuvir tablets (90 mg/400 mg) for 12 weeks, or ledipasvir and sofosbuvir tablets (90 mg/400 mg) + ribavirin for 8 weeks.
Demographics and baseline characteristics were balanced across the treatment groups. Of the 647 treated subjects, the median age was 55 years (range: 20 to 75); 58% of the subjects were male; 78% were White; 19% were Black; 6% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 18 to 56 kg/m2); 81% had baseline HCV RNA levels greater than or equal to 800,000 IU per mL; 80% had genotype 1a HCV infection; 73% had non-C/C IL28B alleles (CT or TT).
Table 11 presents the SVR12 for the ledipasvir and sofosbuvir treatment groups in the ION-3 trial after 8 and 12 weeks of ledipasvir and sofosbuvir treatment. Ribavirin was not shown to increase the SVR12 observed with ledipasvir and sofosbuvir. Therefore, the ledipasvir and sofosbuvir + ribavirin arm is not presented in Table 11.
Table 11 Study ION-3: SVR12 after 8 and 12 Weeks of Treatment in Treatment-Naïve Non-Cirrhotic Subjects with Genotype 1 HCV|
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir | |
|---|---|---|
| ||
|
SVR12 |
94% (202/215) |
96% (208/216) |
|
Outcome for Subjects without SVR | ||
|
On-Treatment Virologic Failure |
0/215 |
0/216 |
|
Relapse* |
5% (11/215) |
1% (3/216) |
|
Other† |
1% (2/215) |
2% (5/216) |
|
SVR by Genotype‡ | ||
|
Genotype 1a |
93% (159/171) |
96% (165/172) |
|
Genotype 1b |
98% (42/43) |
98% (43/44) |
The treatment difference between the 8-week treatment of ledipasvir and sofosbuvir and 12-week treatment of ledipasvir and sofosbuvir was –2.3% (97.5% confidence interval –7.2% to 2.5%). Among subjects with a baseline HCV RNA less than 6 million IU per mL, the SVR12 was 97% (119/123) with 8-week treatment of ledipasvir and sofosbuvir and 96% (126/131) with 12-week treatment of ledipasvir and sofosbuvir.
Relapse rates by baseline viral load are presented in Table 12.
Table 12 Study ION-3: Relapse Rates by Baseline Viral Load after 8 and 12 Weeks of Treatment in Treatment-Naïve Non-Cirrhotic Subjects with Genotype 1 HCV|
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir | |
|---|---|---|
| ||
|
Number of Responders at End of Treatment |
215 |
216 |
|
Baseline HCV RNA* | ||
|
HCV RNA <6 million IU/mL |
2% (2/123) |
2% (2/131) |
|
HCV RNA ≥6 million IU/mL |
10% (9/92) |
1% (1/85) |
Treatment-Naïve Adults with or without Cirrhosis ─ ION-1 (Study 0102)
ION-1 was a randomized, open-label trial that evaluated 12 and 24 weeks of treatment with ledipasvir and sofosbuvir tablets (90 mg/400 mg) with or without ribavirin in 865 treatment-naïve subjects with genotype 1 HCV including those with cirrhosis. Subjects were randomized in a 1:1:1:1 ratio to receive ledipasvir and sofosbuvir for 12 weeks, ledipasvir and sofosbuvir + ribavirin for 12 weeks, ledipasvir and sofosbuvir for 24 weeks, or ledipasvir and sofosbuvir + ribavirin for 24 weeks. Randomization was stratified by the presence or absence of cirrhosis and HCV genotype (1a vs 1b).
Demographics and baseline characteristics were balanced across the treatment groups. Of the 865 treated subjects, the median age was 54 years (range: 18 to 80); 59% of the subjects were male; 85% were White; 12% were Black; 12% were Hispanic or Latino; mean body mass index was 27 kg/m2 (range: 18 to 48 kg/m2); 79% had baseline HCV RNA levels greater than or equal to 800,000 IU per mL; 67% had genotype 1a HCV infection; 70% had non-C/C IL28B alleles (CT or TT); and 16% had cirrhosis.
Table 13 presents the SVR12 for the treatment group of ledipasvir and sofosbuvir for 12 weeks in the ION-1 trial. Ribavirin was not shown to increase SVR12 observed with ledipasvir and sofosbuvir. Therefore, the ledipasvir and sofosbuvir + ribavirin arm is not presented in Table 13.
Table 13 Study ION-1: SVR12 after 12 Weeks of Treatment in Treatment- Naïve Subjects with Genotype 1 HCV with and without Cirrhosis|
Ledipasvir and Sofosbuvir | |
|---|---|
| |
|
SVR12* |
99% (210/213) |
|
Outcome for Subjects without SVR | |
|
On-Treatment Virologic Failure* |
0/213 |
|
Relapse*,† |
<1% (1/212) |
|
Other*,‡ |
1% (2/213) |
SVR12 for selected subgroups are presented in Table 14.
Table 14 Study ION-1: SVR12 for Selected Subgroups after 12 Weeks of Treatment in Treatment-Naïve Subjects with Genotype 1 HCV with and without Cirrhosis|
Ledipasvir and Sofosbuvir 12 Weeks (N=214) | |
|---|---|
| |
|
Genotype* | |
|
Genotype 1a |
98% (142/145) |
|
Genotype 1b |
100% (67/67) |
|
Cirrhosis† | |
|
No |
99% (176/177) |
|
Yes |
94% (32/34) |
Previously-Treated Adults with or without Cirrhosis ─ ION-2 (Study 0109)
ION-2 was a randomized, open-label trial that evaluated 12 and 24 weeks of treatment with ledipasvir and sofosbuvir tablets (90 mg/400 mg) with or without ribavirin in genotype 1 HCV-infected subjects with or without cirrhosis who failed prior therapy with an interferon-based regimen, including regimens containing an HCV protease inhibitor. Subjects were randomized in a 1:1:1:1 ratio to receive ledipasvir and sofosbuvir for 12 weeks, ledipasvir and sofosbuvir + ribavirin for 12 weeks, ledipasvir and sofosbuvir for 24 weeks, or ledipasvir and sofosbuvir + ribavirin for 24 weeks. Randomization was stratified by the presence or absence of cirrhosis, HCV genotype (1a vs 1b) and response to prior HCV therapy (relapse/breakthrough vs nonresponse).
Demographics and baseline characteristics were balanced across the treatment groups. Of the 440 treated subjects, the median age was 57 years (range: 24 to 75); 65% of the subjects were male; 81% were White; 18% were Black; 9% were Hispanic or Latino; mean body mass index was 28 kg/m2 (range: 19 to 50 kg/m2); 89% had baseline HCV RNA levels greater than or equal to 800,000 IU per mL; 79% had genotype 1a HCV infection; 88% had non-C/C IL28B alleles (CT or TT); and 20% had cirrhosis. Forty-seven percent (47%) of the subjects failed a prior therapy of pegylated interferon and ribavirin. Among these subjects, 49% were relapse/breakthrough and 51% were non-responder. Fifty-three percent (53%) of the subjects failed a prior therapy of pegylated interferon and ribavirin with an HCV protease inhibitor. Among these subjects, 62% were relapse/breakthrough and 38% were non-responder.
Table 15 presents the SVR12 for the ledipasvir and sofosbuvir treatment groups in the ION-2 trial. Ribavirin was not shown to increase SVR12 observed with ledipasvir and sofosbuvir. Therefore, the ledipasvir and sofosbuvir + ribavirin arms are not presented in Table 15.
Table 15 Study ION-2: SVR12 after 12 and 24 Weeks of Treatment in Subjects with Genotype 1 HCV with or without Cirrhosis Who Failed Prior Therapy|
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir | |
|---|---|---|
| ||
|
SVR12 |
94% (102/109) |
99% (108/109) |
|
Outcome for Subjects without SVR | ||
|
On-Treatment Virologic Failure |
0/109 |
0/109 |
|
Relapse* |
6% (7/108) |
0/109 |
|
Other† |
0/109 |
1% (1/109) |
Among the subjects with available SVR12 and SVR24 data (206/218), all subjects who achieved SVR12 in the ION-2 study also achieved SVR24.
SVR12 and relapse rates for selected subgroups are presented in Tables 16 and 17.
Table 16 Study ION-2: SVR12 for Selected Subgroups after 12 and 24 Weeks of Treatment in Subjects with Genotype 1 HCV Who Failed Prior Therapy|
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir | |
|---|---|---|
|
RBV = ribavirin. | ||
| ||
|
Genotype | ||
|
Genotype 1a |
95% (82/86) |
99% (84/85) |
|
Genotype 1b |
87% (20/23) |
100% (24/24) |
|
Cirrhosis* | ||
|
No |
95% (83/87) |
99% (85/86) |
|
Yes |
86% (19/22) |
100% (22/22) |
|
Prior HCV Therapy | ||
|
Peg-IFN + RBV |
93% (40/43) |
100% (58/58) |
|
HCV protease inhibitor + Peg-IFN + RBV |
94% (62/66) |
98% (49/50) |
|
Response to Prior HCV Therapy | ||
|
Relapse/Breakthrough |
95% (57/60) |
100% (60/60) |
|
Non-responder |
92% (45/49) |
98% (48/49) |
|
Ledipasvir and Sofosbuvir |
Ledipasvir and Sofosbuvir | |
|---|---|---|
| ||
|
Number of Responders at End of Treatment |
108 |
109 |
|
Cirrhosis* | ||
|
No |
5% (4/86)† |
0% (0/86) |
|
Yes |
14% (3/22) |
0% (0/22) |
|
Presence of Baseline NS5A Resistance-Associated Polymorphisms‡ | ||
|
No |
2% (2/85) |
0% (0/90) |
|
Yes |
22% (5/23) |
0% (0/19) |
|
IL28B Status | ||
|
C/C |
0% (0/10) |
0% (0/16) |
|
Non-C/C |
7% (7/98) |
0% (0/93) |
Previously-Treated Adults with Cirrhosis ─ SIRIUS (Study 0121)
SIRIUS was a randomized, double-blind and placebo-controlled trial that evaluated the efficacy of ledipasvir and sofosbuvir tablets (90 mg/400 mg) + ribavirin for 12 weeks or ledipasvir and sofosbuvir tablets (90 mg/400 mg) without ribavirin for 24 weeks in genotype 1 HCV-infected subjects with compensated cirrhosis who failed prior therapy with a Peg-IFN + ribavirin regimen followed by a subsequent Peg-IFN + ribavirin + an HCV protease inhibitor regimen. Subjects were randomized in a 1:1 ratio to receive placebo for 12 weeks followed by ledipasvir and sofosbuvir + ribavirin for 12 weeks or ledipasvir and sofosbuvir for 24 weeks. Randomization was stratified by HCV genotype (1a vs 1b) and response to prior HCV therapy (never achieved HCV RNA less than LLOQ vs achieved HCV RNA less than LLOQ).
Demographics and baseline characteristics were balanced across the treatment groups. Of the 155 randomized subjects, the median age was 56 years (range: 23 to 77); 74% of the subjects were male; 97% were White; mean body mass index was 27 kg/m2 (range: 19 to 47 kg/m2); 63% had genotype 1a HCV infection; 94% had non-C/C IL28B alleles (CT or TT). One subject discontinued therapy while on placebo, and was not included in the efficacy analysis.
The SVR12 was 96% (74/77) and 97% (75/77) in subjects treated with ledipasvir and sofosbuvir + ribavirin for 12 weeks and ledipasvir and sofosbuvir for 24 weeks without ribavirin, respectively. All 5 subjects who did not achieve SVR12 relapsed.
14.3 Clinical Trials in Subjects with Genotype 4, 5, or 6 HCV
Below are trial descriptions, SVR12 and relapse data in the genotype 4, 5, and 6 HCV populations. Trial results in the genotype 4, 5, and 6 HCV populations are based upon limited number of subjects in some subgroups, particularly in subjects who have been previously treated and subjects with cirrhosis.
Genotype 4
In two open-label studies (Study 1119 and ION-4), ledipasvir and sofosbuvir tablets (90 mg/400 mg) were administered for 12 weeks to treatment-naïve and previously-treated adult subjects with genotype 4 HCV infection. Study 1119 enrolled 44 treatment-naïve or previously-treated subjects with genotype 4 HCV, with or without cirrhosis. ION-4 enrolled 4 treatment-naïve and 4 previously-treated subjects with genotype 4 HCV infection who were coinfected with HIV-1, none of whom had cirrhosis.
In Study 1119, the overall SVR12 rate was 93% (41/44). SVR12 was similar based upon prior HCV treatment history and cirrhosis status. In ION-4, all 8 subjects achieved SVR12.
Genotype 5
In the open-label 1119 trial, ledipasvir and sofosbuvir tablets (90 mg/400 mg) were administered for 12 weeks to 41 treatment-naïve or previously treated adult subjects with genotype 5 HCV infection, with or without cirrhosis. The overall SVR12 was 93% (38/41). SVR12 was similar based upon prior HCV treatment history and cirrhosis status.
Genotype 6
In the open-label ELECTRON-2 trial, ledipasvir and sofosbuvir tablets (90 mg/400 mg) were administered for 12 weeks to 25 treatment-naïve or previously treated adult subjects with genotype 6 HCV infection, with or without cirrhosis. The overall SVR12 was 96% (24/25). SVR12 was similar based upon prior HCV treatment history and cirrhosis status. The single subject who relapsed discontinued study treatment early (at approximately Week 8).
14.4 Clinical Trials in Subjects Coinfected with HCV and HIV-1
ION-4 was an open-label clinical trial that evaluated the safety and efficacy of 12 weeks of treatment with ledipasvir and sofosbuvir tablets (90 mg/400 mg) without ribavirin in HCV treatment-naïve and previously treated adult subjects with genotype 1 or 4 HCV infection who were coinfected with HIV-1. Treatment- experienced subjects had failed prior treatment with Peg-IFN + ribavirin, Peg- IFN + ribavirin + an HCV protease inhibitor, or sofosbuvir + ribavirin. Subjects were on a stable HIV-1 antiretroviral therapy that included emtricitabine + tenofovir disoproxil fumarate, administered with efavirenz, rilpivirine, or raltegravir.
Of the 335 treated subjects, the median age was 52 years (range: 26 to 72); 82% of the subjects were male; 61% were White; 34% were Black; mean body mass index was 27 kg/m2 (range: 18 to 66 kg/m2); 75% had genotype 1a HCV infection; 2% had genotype 4 infection; 76% had non-C/C IL28B alleles (CT or TT); and 20% had compensated cirrhosis. Fifty-five percent (55%) of the subjects were treatment-experienced.
Table 18 presents the SVR12 in the ION-4 trial after 12 weeks of ledipasvir and sofosbuvir treatment.
Table 18 Study ION-4: SVR12 in Subjects with Genotype 1 or 4 HCV Coinfected with HIV-1|
Ledipasvir and Sofosbuvir 12 Weeks | |
|---|---|
| |
|
SVR12 |
96% (321/335) |
|
Outcome for Subjects without SVR | |
|
On-Treatment Virologic Failure |
<1% (2/335) |
|
Relapse* |
3% (10/333) |
|
Other† |
<1% (2/335) |
SVR12 rates were 94% (63/67) in subjects with cirrhosis and 98% (46/47) in subjects who were previously treated and had cirrhosis. The relapse rate in the ION-4 trial in Black subjects was 9% (10/115), all of whom were IL28B non- CC genotype, and none in non-Black subjects (0/220). In the ION-1, ION-2, and ION-3 HCV mono-infection studies, relapse rates were 3% (10/305) in Black subjects and 2% (26/1637) in non-Black subjects.
No subject had HIV-1 rebound during the study. The percentage of CD4+ cells did not change during treatment. Median CD4+ cell count increase of 29 cells/mm3 was observed at the end of treatment with ledipasvir and sofosbuvir tablets for 12 weeks.
14.5 Clinical Trials in Liver Transplant Recipients and/or Subjects with
Decompensated Cirrhosis
SOLAR-1 and SOLAR-2 were two open-label trials that evaluated 12 and 24 weeks of treatment with ledipasvir and sofosbuvir tablets (90 mg/400 mg) in combination with ribavirin in HCV treatment-naïve and previously treated adult subjects with genotype 1 and 4 infection who had undergone liver transplantation and/or who had decompensated liver disease. The two trials were identical in study design. Subjects were enrolled in one of the seven groups in the trials based on liver transplantation status and severity of hepatic impairment (see Table 19). Subjects with a CPT score greater than 12 were excluded. Within each group, subjects were randomized in a 1:1 ratio to receive ledipasvir and sofosbuvir + ribavirin for 12 weeks or ledipasvir and sofosbuvir + ribavirin for 24 weeks. For subjects with decompensated cirrhosis in SOLAR-1 and SOLAR-2 studies, the starting ribavirin dosage was 600 mg per day regardless of transplantation status. Ribavirin dose adjustments were performed according to the ribavirin labeling [see Clinical Studies (14.1)].
Demographics and baseline characteristics were balanced across the treatment groups. Of the 670 treated subjects, the median age was 59 years (range: 21 to 81); 77% of the subjects were male; 91% were White; mean body mass index was 28 kg/m2 (range: 18 to 49 kg/m2); 94% and 6% had genotype 1 and 4 HCV infection, respectively; 78% of the subjects failed a prior HCV therapy.
Table 19 presents the pooled SVR12 rates for SOLAR-1 and SOLAR-2 in subjects with genotype 1 HCV treated with ledipasvir and sofosbuvir + ribavirin for 12 weeks. The SVR12 rates observed with 24 weeks of ledipasvir and sofosbuvir + ribavirin were similar to the SVR12 rates observed with 12 weeks of treatment. Therefore, the results for the ledipasvir and sofosbuvir + ribavirin 24 weeks arm are not presented in Table 19.
Table 19 Studies SOLAR-1 and SOLAR-2: SVR12 and Relapse Rates After 12 Weeks of Treatment with Ledipasvir and Sofosbuvir and Ribavirin in Subjects with Genotype 1 HCV Who Were Post Liver Transplant and/or Who Had Decompensated Liver Disease|
Ledipasvir and Sofosbuvir + RBV 12 weeks | ||
|---|---|---|
|
SVR12 (N=300)*,† |
Relapse (N=288)*,†,‡ | |
| ||
|
Pre-transplant | ||
|
CPT B |
87% (45/52) |
12% (6/51) |
|
CPT C |
88% (35/40) |
5% (2/37) |
|
Post-transplant | ||
|
Metavir score F0–F3 |
95% (94/99) |
3% (3/97) |
|
CPT A |
98% (55/56) |
0% (0/55) |
|
CPT B |
89% (41/46) |
2% (1/42) |
|
CPT C |
57% (4/7) |
33% (2/6) |
There were 7 subjects with fibrosing cholestatic hepatitis in the 12-week treatment arm, and all subjects achieved SVR12.
In genotype 4 HCV post-transplant subjects without cirrhosis or with compensated cirrhosis treated with ledipasvir and sofosbuvir + ribavirin for 12 weeks (N=12), the SVR12 rate was similar to rates reported with genotype 1; no subjects relapsed. Available data in subjects with genotype 4 HCV who had decompensated cirrhosis (pre- and post-liver transplantation) were insufficient for dosing recommendations; therefore, these results are not presented.
14.6 Clinical Trials in Adults with Severe Renal Impairment, Including
those Requiring Dialysis
Trial 0154 was an open-label clinical trial that evaluated 12 weeks of treatment with ledipasvir and sofosbuvir tablets (90 mg/400 mg) in 18 treatment-naïve and treatment-experienced (subjects with prior exposure to an HCV NS5B polymerase inhibitor were excluded) genotype 1 HCV-infected adults with severe renal impairment not requiring dialysis. At baseline, two subjects (11%) had cirrhosis and the mean eGFR was 24.9 mL/min (range: 9.0 to 39.6). The SVR rate was 100% (18/18).
As shown in the table below, Trial 4063 was an open-label three-arm clinical trial that evaluated 8, 12, and 24 weeks of treatment with ledipasvir and sofosbuvir in a total of 63 adults with chronic HCV infection and ESRD requiring dialysis. Of the 63 subjects, 10% had cirrhosis, 24% were treatment- experienced, 95% were on hemodialysis, and 5% were on peritoneal dialysis; mean duration on dialysis was 12 years (range: 0.2 to 43 years). The SVR rates for the 8, 12, and 24 week ledipasvir and sofosbuvir treatment groups are shown in Table 20.
Table 20 Trial 4063: SVR12 after 8, 12, and 24 Weeks of Treatment in Adults with HCV with or without Cirrhosis and with Severe Renal Impairment Requiring Dialysis|
Ledipasvir and sofosbuvir 8 Weeks |
Ledipasvir and sofosbuvir 12 Weeks |
Ledipasvir and sofosbuvir 24 Weeks | |
|---|---|---|---|
| |||
|
Population |
Treatment-naïve, GT 1 HCV |
Treatment-naïve and treatment-experienced* GT 1, 5, 6† HCV |
Treatment-experienced, GT 1 HCV with compensated cirrhosis |
|
SVR12 |
93% (42/45) |
100% (12/12) |
83% (5/6) |
|
Outcome for Subjects without SVR | |||
|
On-Treatment Virologic Failure |
0/45 |
0/12 |
0/6 |
|
Relapse |
0/44 |
0/12 |
0/6 |
|
Other‡ |
7% (3/45) |
0/12 |
17% (1/6) |
14.7 Clinical Trial in Pediatric Subjects
The efficacy of ledipasvir and sofosbuvir was evaluated in an open-label trial (Study 1116) in 224 HCV treatment-naïve (N=186) and treatment-experienced (N=38) pediatric subjects 3 years of age or older. This study evaluated 12 weeks of treatment with ledipasvir and sofosbuvir once daily in genotype 1 (N=183) or genotype 4 (N=3) treatment-naive subjects without cirrhosis or with compensated cirrhosis; genotype 1 treatment-experienced subjects without cirrhosis (N=37); and evaluated 24 weeks of treatment with ledipasvir and sofosbuvir once daily in one genotype 1 subject who was both treatment- experienced and cirrhotic.
Subjects 12 Years to <18 Years of Age: Ledipasvir and sofosbuvir was evaluated in 100 subjects 12 years to <18 years of age with HCV genotype 1 infection. Demographics and baseline characteristics were balanced across treatment-naïve and treatment-experienced subjects (patients had failed an interferon based regimen with or without ribavirin). The median age was 15 years (range: 12 to 17); 63% of the subjects were female; 91% were White, 7% were Black, and 2% were Asian; 13% were Hispanic/Latino; mean body mass index was 23 kg/m2 (range: 13.1 to 36.6 kg/m2); mean weight was 61 kg (range 33 to 126 kg); 55% had baseline HCV RNA levels greater than or equal to 800,000 IU/mL; 81% had genotype 1a HCV infection. One subject (treatment-naïve) had known compensated cirrhosis. The majority of subjects (84%) had been infected through vertical transmission.
The SVR12 rate was 98% overall (98% [78/80] in treatment-naïve subjects and 100% [20/20] in treatment-experienced subjects). No subject experienced on- treatment virologic failure or relapse. Two subjects were lost to follow-up.
Subjects 6 Years to <12 Years of Age: Ledipasvir and sofosbuvir was evaluated in 90 subjects 6 years to <12 years of age with HCV genotype 1 or 4 infection. Among these subjects, 72 (80%) were treatment-naïve and 18 (20%) were treatment-experienced. Eighty-nine of the subjects (87 with genotype 1 HCV infection and 2 with genotype 4 HCV infection) were treated with ledipasvir and sofosbuvir for 12 weeks, 1 subject with genotype 1 HCV infection was treated with ledipasvir and sofosbuvir for 24 weeks. The median age was 9 years (range: 6 to 11); 59% of the subjects were male; 79% were White, 8% were Black, and 6% were Asian; 10% were Hispanic/Latino; mean body mass index was 18 kg/m2 (range: 13 to 31kg/m2); mean weight was 33 kg (range 18 to 76 kg); 59% had baseline HCV RNA levels greater than or equal to 800,000 IU/mL; 86% had genotype 1a HCV infection; 2 subjects (1 treatment-naïve, 1 treatment- experienced) had known compensated cirrhosis. The majority of subjects (97%) had been infected through vertical transmission.
The SVR12 rate was 99% (86/87) in subjects with genotype 1 HCV infection, and 100% (2/2) in subjects with genotype 4 HCV infection. The one genotype 1 subject treated with ledipasvir and sofosbuvir for 24 weeks also achieved SVR12. The one subject (genotype 1) who did not achieve SVR12 and relapsed had been treated with ledipasvir and sofosbuvir for 12 weeks.
Subjects 3 Years to <6 Years of Age: Ledipasvir and sofosbuvir was evaluated in 34 subjects 3 years to <6 years of age with HCV genotype 1 (N = 33) or genotype 4 (N = 1) infection. All of the subjects were treatment-naïve and treated with ledipasvir and sofosbuvir for 12 weeks. The median age was 5 years (range: 3 to 5); 71% of the subjects were female; 79% were White, 3% were Black, and 6% were Asian; 18% were Hispanic/Latino; mean body mass index was 17 kg/m2 (range: 13 to 25 kg/m2); mean weight was 19 kg (range 11 to 34 kg); 56% had baseline HCV RNA levels greater than or equal to 800,000 IU/mL; 82% had genotype 1a HCV infection; no subjects had known cirrhosis. All subjects (100%) had been infected through vertical transmission.
The SVR12 rate was 97% (32/33) in subjects with genotype 1 HCV infection, and the one subject with genotype 4 HCV infection also achieved SVR12. One subject prematurely discontinued study treatment due to an adverse event.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Ledipasvir and sofosbuvir tablets (90 mg/400 mg) are orange, diamond-shaped, film-coated, debossed with "ASE" on one side and "9875" on the other side of the tablet. Each carton contains 28 tablets (2 blister cards each containing 14 tablets) (NDC 72626-2601-1).
Store at room temperature below 30 °C (86 °F).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
Inform patients that HBV reactivation can occur in patients coinfected with HBV during or after treatment of HCV infection. Advise patients to tell their healthcare provider if they have a history of HBV infection [see Warnings and Precautions (5.1)].
Serious Symptomatic Bradycardia When Coadministered with Amiodarone
Advise patients to seek medical evaluation immediately for symptoms of bradycardia such as near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pain, confusion, or memory problems [see Warnings and Precautions (5.2), Adverse Reactions (6.2), and Drug Interactions (7.2)].
Drug Interactions
Inform patients that ledipasvir and sofosbuvir may interact with other drugs. Advise patients to report to their healthcare provider the use of any other prescription or nonprescription medication or herbal products including St. John's wort [see Warnings and Precautions (5.2, 5.3) and Drug Interactions (7)].
Pregnancy
Advise patients to avoid pregnancy during combination treatment with ledipasvir and sofosbuvir and ribavirin and for 6 months after completion of treatment. Inform patients to notify their healthcare provider immediately in the event of a pregnancy [see Use in Specific Populations (8.1)].
Hepatitis C Virus Transmission
Inform patients that the effect of treatment of hepatitis C infection on transmission is not known, and that appropriate precautions to prevent transmission of the hepatitis C virus during treatment or in the event of treatment failure should be taken.
Administration
Advise patients to take ledipasvir and sofosbuvir every day at the regularly scheduled time with or without food. Inform patients that it is important not to miss or skip doses and to take ledipasvir and sofosbuvir for the duration that is recommended by the physician.
For ledipasvir and sofosbuvir (HARVONI) oral pellets, advise patients or caregivers to read and follow the Instructions for Use for preparing the correct dose.
SPL UNCLASSIFIED SECTION
Manufactured for:
Asegua Therapeutics LLC
An affiliate of Gilead Sciences, Inc.
Foster City, CA 94404
Asegua is a trademark of Asegua Therapeutics LLC. All other trademarks referenced herein are the property of their respective owners.
205834-AG-011
SPL PATIENT PACKAGE INSERT SECTION
|
This Patient Information has been approved by the U.S. Food and Drug Administration |
Revised: 03/2020 | ||
|
Patient Information | |||
|
Important: If you take ledipasvir and sofosbuvir with ribavirin, you should also read the Medication Guide for ribavirin. | |||
|
What is the most important information I should know about ledipasvir and
sofosbuvir? | |||
|
What is ledipasvir and sofosbuvir?
It is not known if ledipasvir and sofosbuvir is safe and effective in children with HCV under 3 years of age. | |||
|
Before taking ledipasvir and sofosbuvir, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Ledipasvir and sofosbuvir and other medicines may affect each other. This can cause you to have too much or not enough ledipasvir and sofosbuvir or other medicines in your body. This may affect the way ledipasvir and sofosbuvir or your other medicines work, or may cause side effects.Keep a list of your medicines to show your healthcare provider and pharmacist.
Do not start taking a new medicine without telling your healthcare provider. Your healthcare provider can tell you if it is safe to take ledipasvir and sofosbuvir with other medicines. | |||
|
How should I take ledipasvir and sofosbuvir?
If you take too much ledipasvir and sofosbuvir, call your healthcare provider or go to the nearest hospital emergency room right away. | |||
|
How should I give ledipasvir and sofosbuvir (HARVONI) oral pellets to my child? | |||
|
See the detailed Instructions for Use for information about how to give or take a dose of ledipasvir and sofosbuvir (HARVONI) oral pellets.
| |||
|
What are the possible side effects of ledipasvir and sofosbuvir? *Hepatitis B virus reactivation. See "What is the most important information I should know about ledipasvir and sofosbuvir?" *Slow heart rate (bradycardia). Ledipasvir and sofosbuvir treatment may result in slowing of the heart rate along with other symptoms when taken with amiodarone (Cordarone®, Nexterone®, Pacerone®), a medicine used to treat certain heart problems. In some cases bradycardia has led to death or the need for a heart pacemaker when amiodarone is taken with ledipasvir and sofosbuvir tablets. Get medical help right away if you take amiodarone with ledipasvir and sofosbuvir tablets and get any of the following symptoms: | |||
|
|
| |
|
The most common side effects of ledipasvir and sofosbuvir include: | |||
|
|
| |
|
These are not all the possible side effects of ledipasvir and sofosbuvir. For
more information, ask your healthcare provider or pharmacist. | |||
|
How should I store ledipasvir and sofosbuvir tablets?
Keep ledipasvir and sofosbuvir tablets and all medicines out of the reach of children. | |||
|
General information about the safe and effective use of ledipasvir and
sofosbuvir | |||
|
What are the ingredients in ledipasvir and sofosbuvir tablets (90 mg/400
mg)? |
