Daraprim
DARAPRIM (pyrimethamine)
f634f34b-0952-4dd3-9da3-f84b6ab823de
HUMAN PRESCRIPTION DRUG LABEL
Dec 7, 2023
TILDE Sciences LLC
DUNS: 119033490
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
pyrimethamine
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
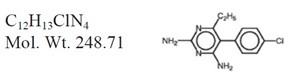
DARAPRIM (pyrimethamine) is an antiparasitic compound available in tablet form for oral administration. Each scored tablet contains 25 mg pyrimethamine and the inactive ingredients corn and potato starch, lactose, and magnesium stearate.
Pyrimethamine, known chemically as 5-(4- chlorophenyl)-6-ethyl-2, 4-pyrimidinediamine, has the following structural formula: