JAYSUING Pain Relief Knee Patches
358a3e81-abb5-0a65-e063-6294a90a3d69
HUMAN OTC DRUG LABEL
May 19, 2025
Shantou Jaysuing Cleaning and Environmental Protection Technology Co., Ltd.
DUNS: 617522260
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GLYCERIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
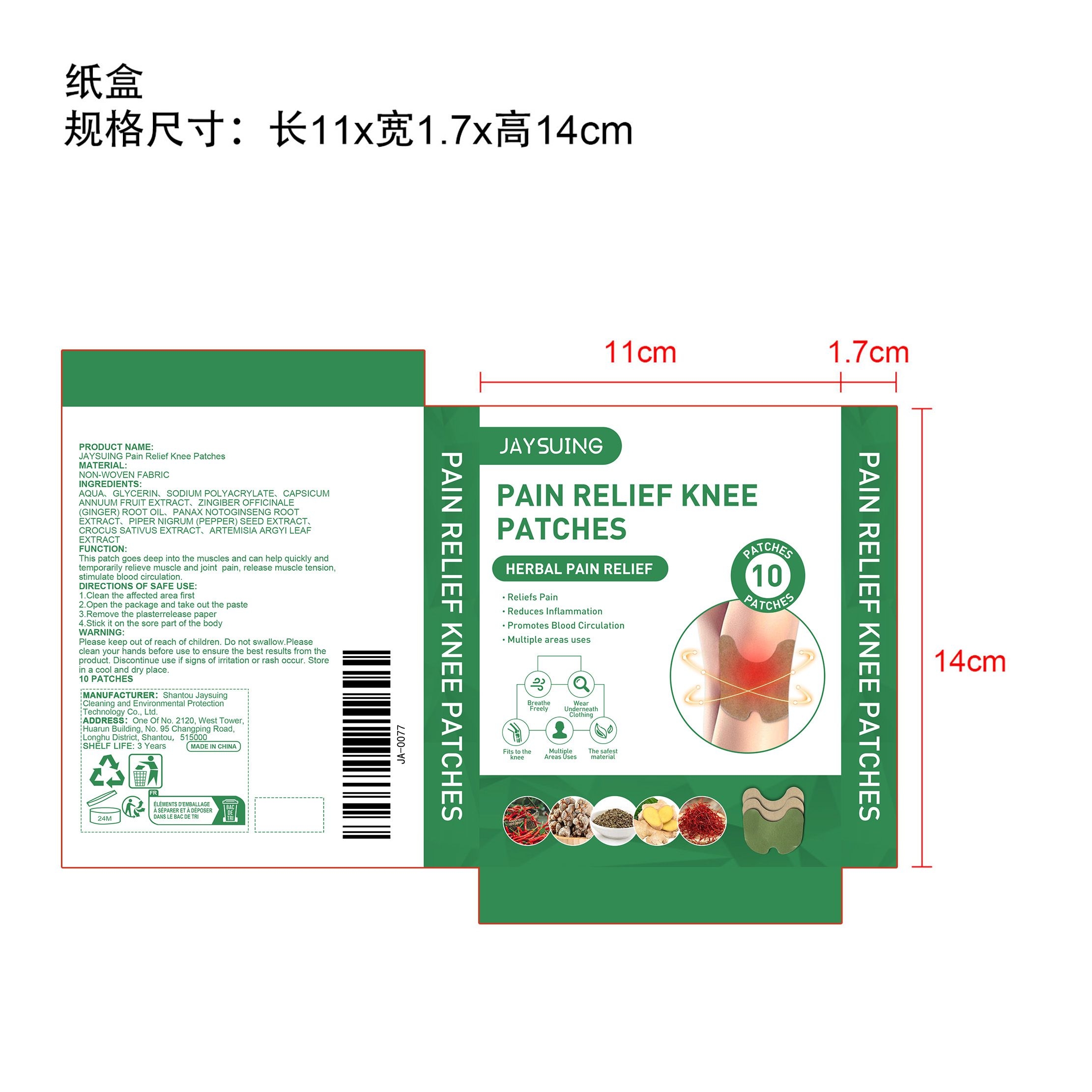
INDICATIONS & USAGE SECTION
OTC - ACTIVE INGREDIENT SECTION
CAPSICUM ANNUUM FRUIT EXTRACT、
ZINGIBER OFFICINALE (GINGER) ROOT OIL、
PANAX NOTOGINSENG ROOT EXTRACT、
PIPER NIGRUM (PEPPER) SEED EXTRACT、
CROCUS SATIVUS EXTRACT、
ARTEMISIA ARGYI LEAF EXTRACT
OTC - PURPOSE SECTION
Reliefs Pain
Reduces Inflammation
Promotes Blood Circulation
Multiple areas uses
WARNINGS SECTION
Please keep out of reach of children. Do not swallow.Please clean your hands before use to ensure the best results from the product. Discontinue use if signs of irritation or rash occur. Store in a cool and dry place.
OTC - WHEN USING SECTION
1.Clean the affected area first
2.Open the package and take out the paste
3.Remove the plasterrelease paper
4.Stick it on the sore part of the body
OTC - DO NOT USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - STOP USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Please keep out of reach of children. Do not swallow.
STORAGE AND HANDLING SECTION
Store in a cool and dry place.
INACTIVE INGREDIENT SECTION
AQUA、
GLYCERIN、
SODIUM POLYACRYLATE
