Norethindrone Acetate
Norethindrone Acetate Tablets, USP Rx Only
827c7ef1-a8b7-d0c4-b062-c5154b331a35
HUMAN PRESCRIPTION DRUG LABEL
Sep 17, 2025
AvKARE
DUNS: 796560394
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Norethindrone
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL

ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
SeeWARNINGS andPRECAUTIONS.
The following adverse reactions have been observed in women taking progestins:
- Breakthrough bleeding
- Spotting
- Change in menstrual flow
- Amenorrhea
- Edema
- Changes in weight (decreases, increases)
- Changes in the cervical squamo-columnar junction and cervical secretions
- Cholestatic jaundice
- Rash (allergic) with and without pruritus
- Melasma or chloasma
- Clinical depression
- Acne
- Breast enlargement/tenderness
- Headache/migraine
- Urticaria
- Abnormalities of liver tests (i.e., AST, ALT, Bilirubin)
- Decreased HDL cholesterol and increased LDL/HDL ratio
- Mood swings
- Nausea
- Insomnia
- Anaphylactic/anaphylactoid reactions
- Thrombotic and thromboembolic events (e.g., deep vein thrombosis, pulmonary embolism, retinal vascular thrombosis, cerebral thrombosis and embolism)
- Optic neuritis (which may lead to partial or complete loss of vision)
To report SUSPECTED ADVERSE REACTIONS contact AvKARE at 1-855-361-3993; email drugsafety@avkare.com; or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Norethindrone acetate induces secretory changes in an estrogen-primed endometrium. On a weight basis, it is twice as potent as norethindrone.
Pharmacokinetics
Absorption
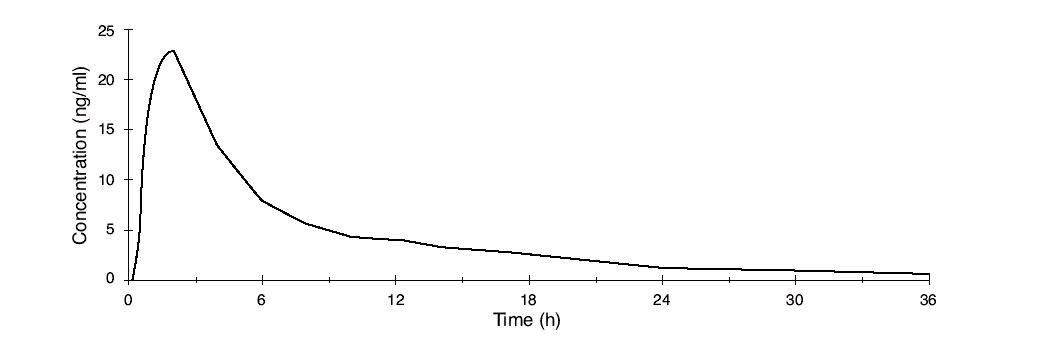
Norethindrone acetate is completely and rapidly deacetylated to norethindrone (NET) after oral administration, and the disposition of norethindrone acetate is indistinguishable from that of orally administered norethindrone. Norethindrone acetate is rapidly absorbed from norethindrone acetate tablets, with maximum plasma concentration of norethindrone generally occurring at about 2 hours post-dose. The pharmacokinetic parameters of norethindrone following single oral administration of norethindrone acetate in 29 healthy female volunteers are summarized in Table 1.
Table 1.Pharmacokinetic Parameters after a Single Dose of Norethindrone Acetate in Healthy Women|
Norethindrone Acetate (n=29) Arithmetic Mean ± SD | |
|
Norethindrone (NET) | |
|
AUC = area under the curve, | |
|
C max = maximum plasma concentration, | |
|
t max = time at maximum plasma concentration, | |
|
t 1/2 = half-life, | |
|
SD = standard deviation | |
|
AUC (0-inf)(ng/ml*h) |
166.90 ± 56.28 |
|
C max (ng/ml) |
26.19 ± 6.19 |
|
t max (h) |
1.83 ± 0.58 |
|
t 1/2 (h) |
8.51 ± 2.19 |
Figure 1. Mean Plasma Concentration Profile after a Single Dose of 5 mg Administered to 29 Healthy Female Volunteers under Fasting Conditions
Effect of Food
The effect of food administration on the pharmacokinetics of norethindrone acetate has not been studied.
Distribution
Norethindrone is 36% bound to sex hormone-binding globulin (SHBG) and 61% bound to albumin. Volume of distribution of norethindrone is about 4 L/kg.
Metabolism
Norethindrone undergoes extensive biotransformation, primarily via reduction, followed by sulfate and glucuronide conjugation. The majority of metabolites in the circulation are sulfates, with glucuronides accounting for most of the urinary metabolites.
Excretion
Plasma clearance value for norethindrone is approximately 0.4 L/hr/kg. Norethindrone is excreted in both urine and feces, primarily as metabolites. The mean terminal elimination half-life of norethindrone following a single dose administration of norethindrone acetate is approximately 9 hours.
Special Populations
Geriatrics
The effect of age on the pharmacokinetics of norethindrone after norethindrone acetate administration has not been evaluated.
Race
The effect of race on the disposition of norethindrone after norethindrone acetate administration has not been evaluated.
Renal Insufficiency
The effect of renal disease on the disposition of norethindrone after norethindrone acetate administration has not been evaluated. In premenopausal women with chronic renal failure undergoing peritoneal dialysis who received multiple doses of an oral contraceptive containing ethinyl estradiol and norethindrone, plasma norethindrone concentration was unchanged compared to concentrations in premenopausal women with normal renal function.
Hepatic Insufficiency
The effect of hepatic disease on the disposition of norethindrone after norethindrone acetate administration has not been evaluated. However, norethindrone acetate is contraindicated in markedly impaired liver function or liver disease.
Drug Interactions
No pharmacokinetic drug interaction studies investigating any drug-drug interactions with norethindrone acetate have been conducted.
DESCRIPTION SECTION
DESCRIPTION
Norethindrone acetate tablets, USP - 5 mg oral tablets.
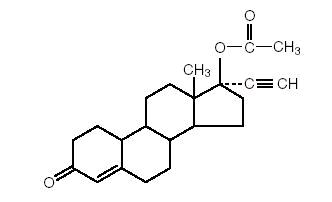
Norethindrone acetate, USP (17-hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate), a synthetic, orally active progestin, is the acetic acid ester of norethindrone, USP. It is a white, or creamy white, crystalline powder.
Norethindrone acetate tablets, USP contain the following inactive ingredients: lactose, magnesium stearate, and microcrystalline cellulose.
HOW SUPPLIED SECTION
HOW SUPPLIED
Norethindrone acetate tablets, USP,5 mg, are supplied as white to off- white oval, biconvex tablets debossed with “AN” bisect “475” on one side and plain on the other side.
They are available as follows:
Bottles of 90: NDC 42291-650-90
Store at 20º to 25ºC (68º to 77ºF) [See USP Controlled Room Temperature].
All registered trademarks in this document are the property of their respective owners.
SPL UNCLASSIFIED SECTION
Manufactured for:
AvKARE
Pulaski, TN 38478
Mfg. Rev. 08-2023-01
AV Rev. 11/24 (A)
