SIMVASTATIN
These highlights do not include all the information needed to use SIMVASTATIN TABLETS safely and effectively. See full prescribing information for SIMVASTATIN TABLETS. SIMVASTATIN tablets, for oral useInitial U.S. Approval: 1991
3e48217f-8175-4e9b-9fd0-909be4bbba95
HUMAN PRESCRIPTION DRUG LABEL
Sep 23, 2025
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 7
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
SIMVASTATIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
SIMVASTATIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
SIMVASTATIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
SIMVASTATIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
SIMVASTATIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
SIMVASTATIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
SIMVASTATIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
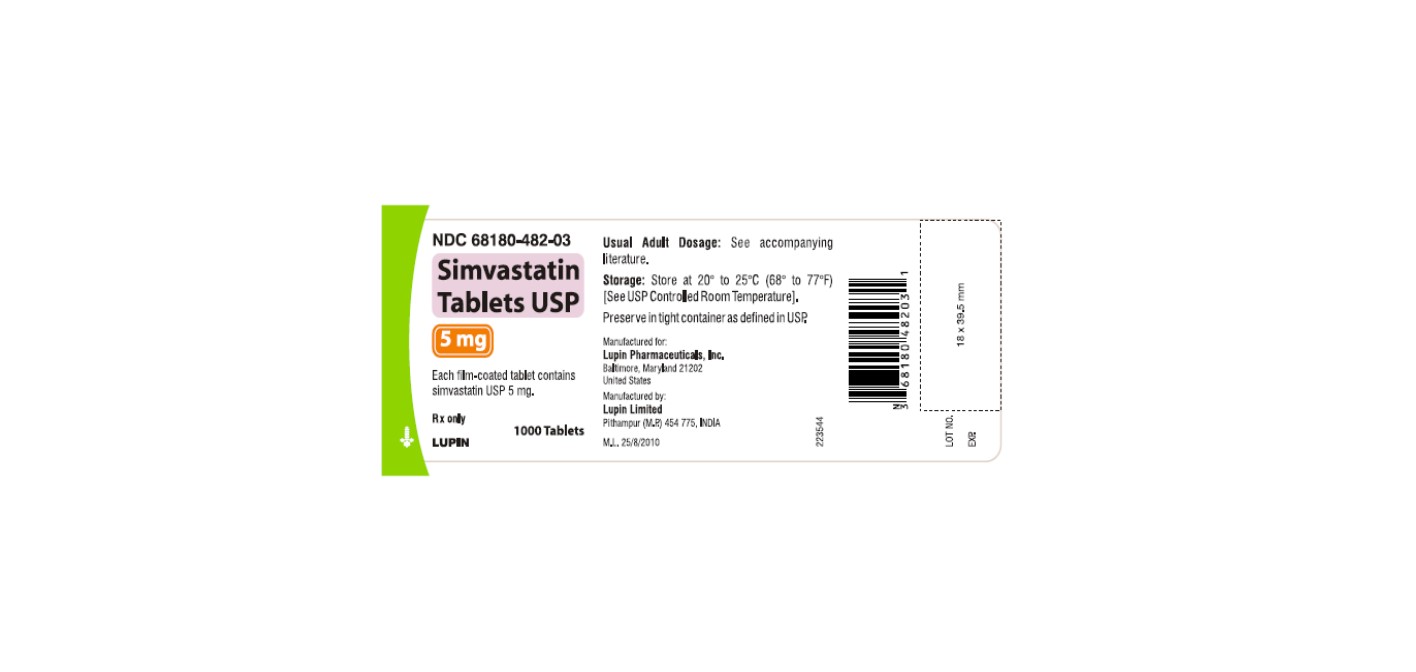
NDC 68180-482-03
Simvastatin Tablets USP 5 mg
Each film-coated tablet contains simvastatin USP 5 mg.
Rx only
1000 Tablets
LUPIN
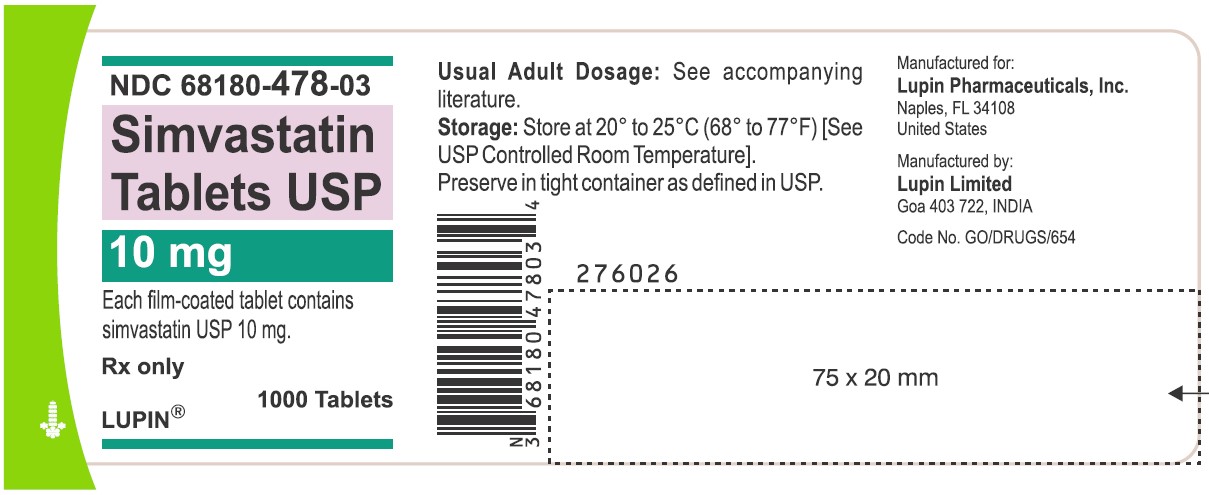
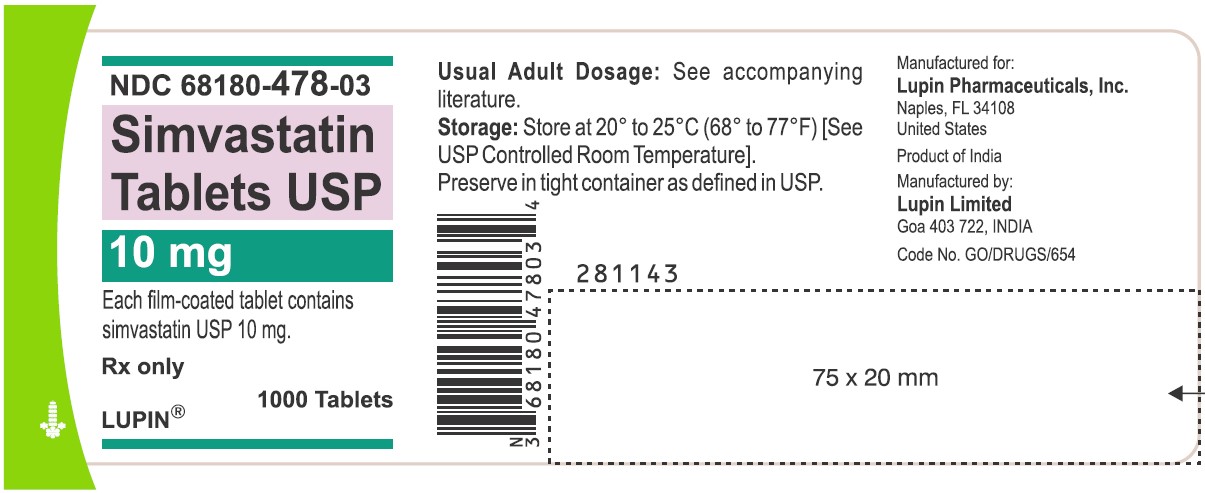
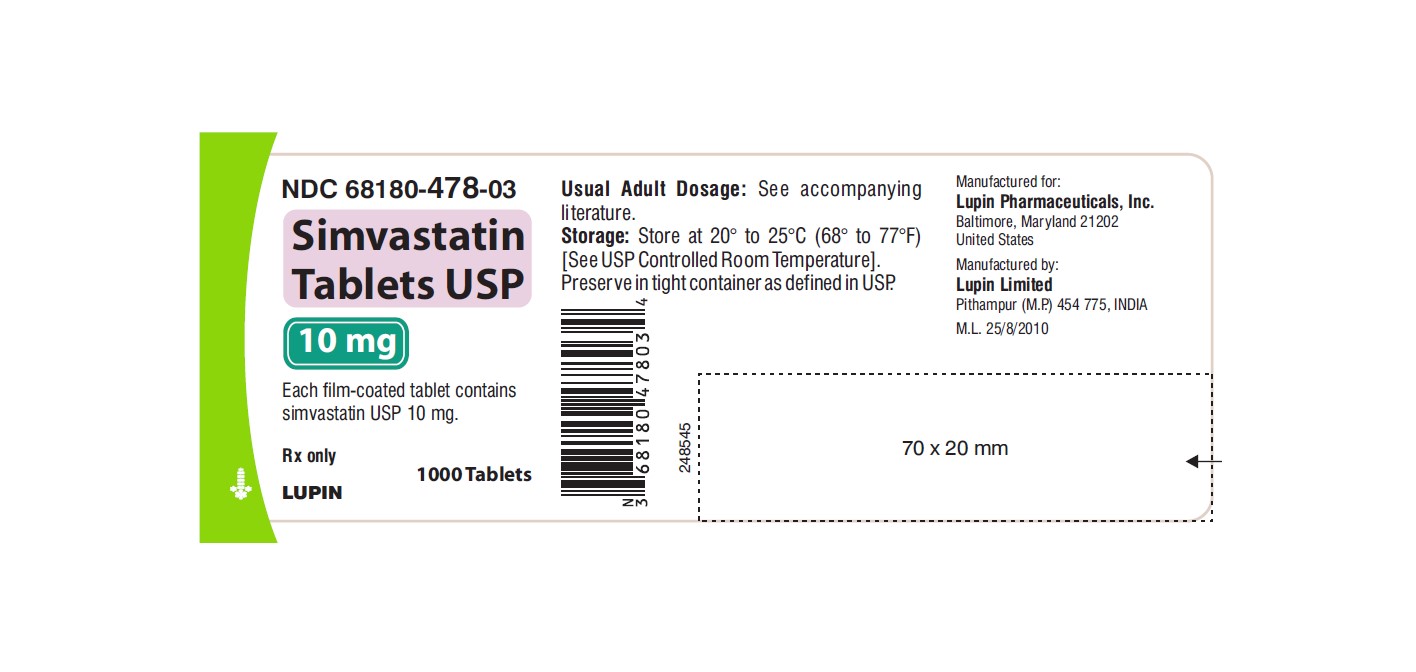
NDC 68180-478-03
Simvastatin Tablets USP 10 mg
Each film-coated tablet contains simvastatin USP 10 mg.
Rx only
1000 Tablets
LUPIN
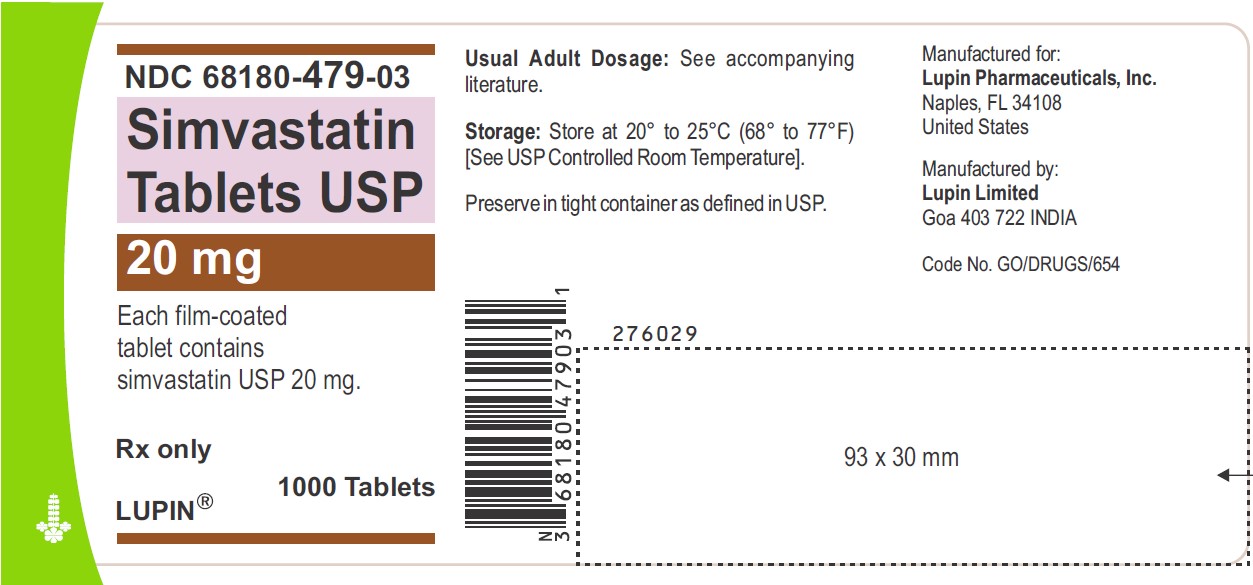
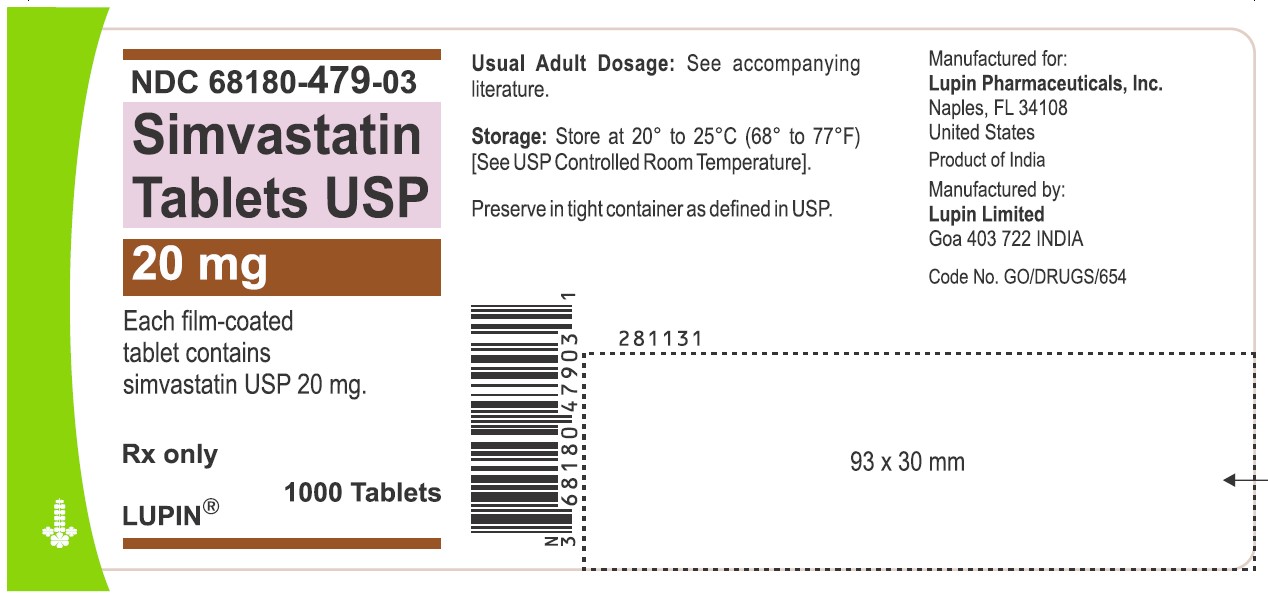
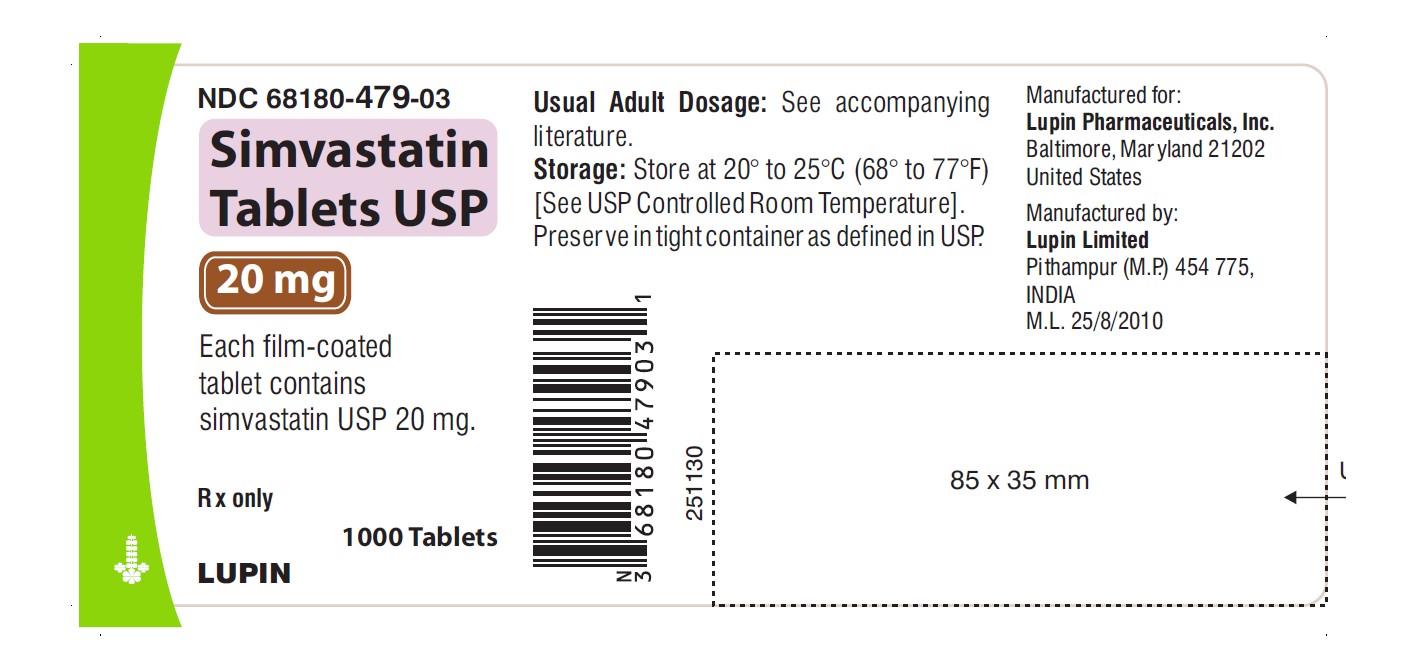
NDC 68180-479-03
Simvastatin Tablets USP 20 mg
Each film-coated tablet contains simvastatin USP 20 mg.
Rx only
1000 Tablets
LUPIN
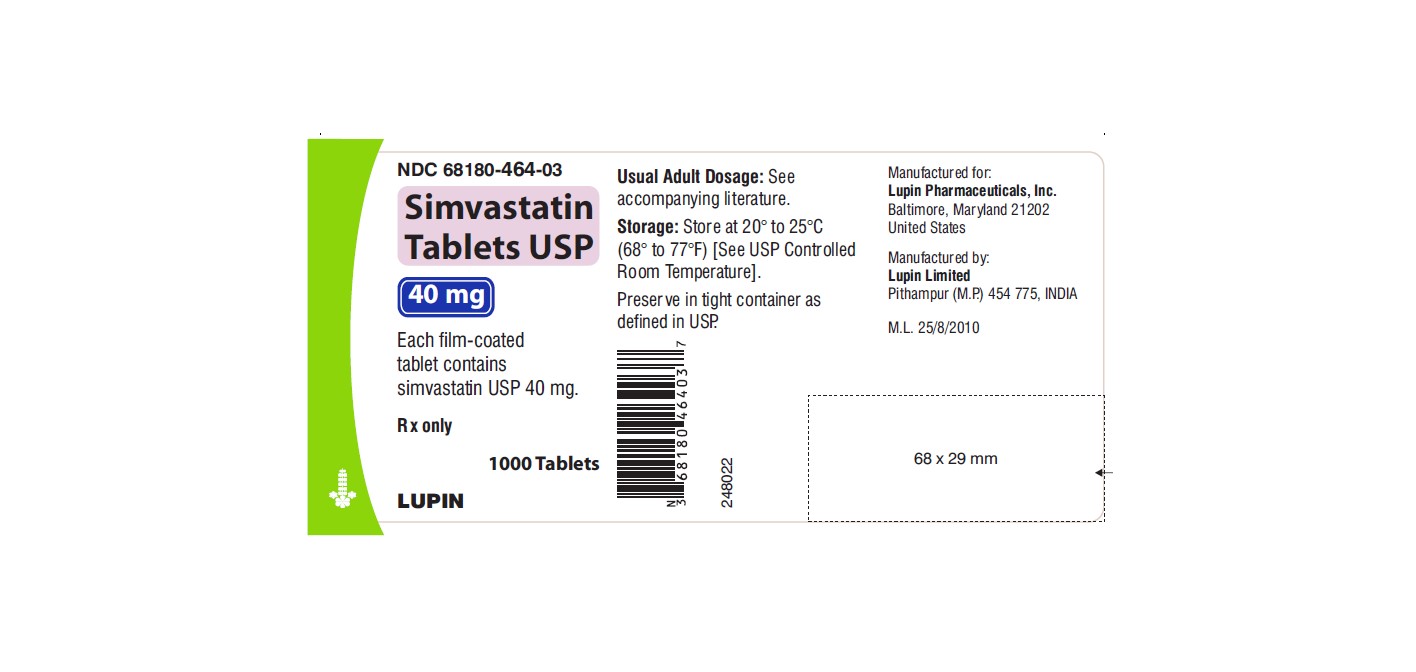
NDC 68180-464-03
Simvastatin Tablets USP 40 mg
Each film-coated tablet contains simvastatin USP 40 mg.
Rx only
1000 Tablets
LUPIN


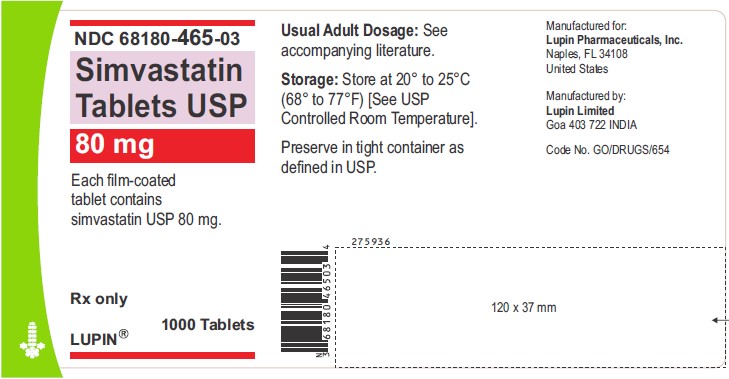
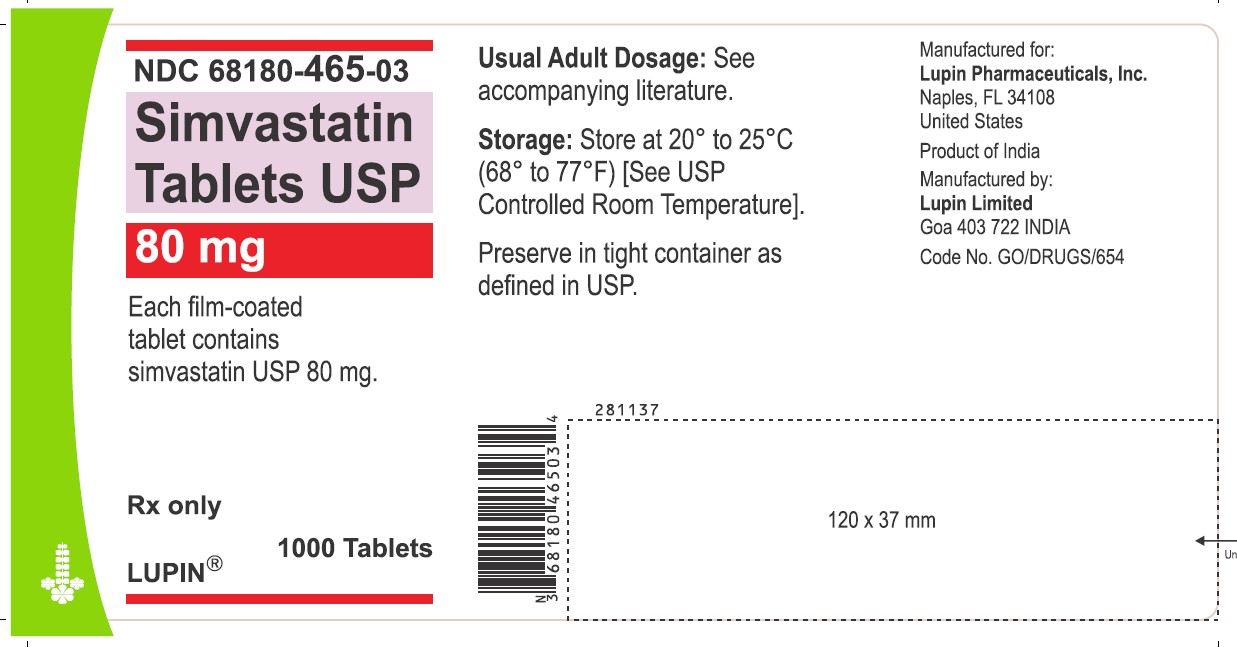
NDC 68180-465-03
Simvastatin Tablets USP 80 mg
Each film-coated tablet contains simvastatin USP 80 mg.
Rx only
1000 Tablets
LUPIN

ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following important adverse reactions are described below and elsewhere in the labeling:
- Myopathy and Rhabdomyolysis [see WARNINGS AND PRECAUTIONS (5.1)]
- Immune-Mediated Necrotizing Myopathy [see WARNINGS AND PRECAUTIONS (5.2)]
- Hepatic Dysfunction [see WARNINGS AND PRECAUTIONS (5.3)]
- Increases in HbA1c and Fasting Serum Glucose Levels [see WARNINGS AND PRECAUTIONS (5.4)]
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In clinical studies, 2,423 adult patients were exposed to simvastatin with a median duration of follow-up of approximately 18 months. The most commonly reported adverse reactions (incidence ≥5%) in these simvastatin clinical studies were: upper respiratory infections (9%), headache (7%), abdominal pain (7%), constipation (7%), and nausea (5%). Overall, 1.4% of patients discontinued simvastatin due to adverse reactions. The most common adverse reactions that led to discontinuation were: gastrointestinal disorders (0.5%), myalgia (0.1%), and arthralgia (0.1%).
In a Cardiovascular Outcomes Study (the Scandinavian Simvastatin Survival Study [Study 4S]), adult patients (age range 35-71 years, 19% women, 100% Caucasians) were treated with 20-40 mg per day of simvastatin or placebo over a median of 5.4 years [see CLINICAL STUDIES (14)]; adverse reactions reported in ≥2% of patients and at a rate greater than placebo are shown in Table 1.
Table 1 : Adverse Reactions Reported ≥2% of Patients Treated with Simvastatin and Greater than Placebo in Study 4S|
% Placebo |
% Simvastatin | |
|
Bronchitis |
6.3 |
6.6 |
|
Abdominal pain |
5.8 |
5.9 |
|
Atrial fibrillation |
5.1 |
5.7 |
|
Gastritis |
3.9 |
4.9 |
|
Eczema |
3.0 |
4.5 |
|
Vertigo |
4.2 |
4.5 |
|
Diabetes mellitus |
3.6 |
4.2 |
|
Insomnia |
3.8 |
4.0 |
|
Myalgia |
3.2 |
3.7 |
|
Urinary tract infection |
3.1 |
3.2 |
|
Edema/swelling |
2.3 |
2.7 |
|
Headache |
2.1 |
2.5 |
|
Sinusitis |
1.8 |
2.3 |
|
Constipation |
1.6 |
2.2 |
Myopathy/Rhabdomyolysis
In clinical studies with a median follow-up of at least 4 years, in which 24,747 patients received simvastatin, the incidence of myopathy (defined as unexplained muscle weakness, pain, or tenderness accompanied by CK increases greater than 10xULN) was approximately 0.03%, 0.08%, and 0.61% for the simvastatin 20 mg, 40 mg, and 80 mg daily groups, respectively.
In a clinical outcomes study in which 12,064 adult patients with a history of myocardial infarction were treated with simvastatin (mean follow-up 6.7 years), the incidence of myopathy (defined as unexplained muscle weakness or pain with a serum CK >10x [1200 U/L] ULN) in patients taking simvastatin 20 mg and 80 mg daily was approximately 0.02% and 0.9%, respectively. The incidence of rhabdomyolysis (defined as myopathy with a CK >40xULN) in patients on simvastatin 20 mg and 80 mg daily was approximately 0% and 0.4%, respectively. The incidence of myopathy and rhabdomyolysis were highest during the first year and then decreased during the subsequent years of treatment.
In another clinical outcomes study in which 10,269 adult patients were treated with simvastatin 40 mg per day (mean follow-up of 5 years), the incidence of myopathy/rhabdomyolysis was <0.1% in patients treated with simvastatin.
Elevations in Liver Enzyme Tests
Moderate (less than 3xULN) elevations of serum transaminases have been reported with use of simvastatin.
Persistent increases to more than 3xULN in serum transaminases have occurred in approximately 1% of patients receiving simvastatin in clinical studies. Marked persistent increases of hepatic transaminases have occurred with simvastatin. Elevated alkaline phosphatase and γ-glutamyl transpeptidase have also been reported.
In Study 4S, with a median follow-up of 5.4 years, 1,986 adult patients were treated with simvastatin 20 mg once daily, of whom 37% titrated to 40 mg once daily. The percentage of patients with one or more occurrences of transaminase elevations to >3xULN was 0.7% in patients taking simvastatin compared with 0.6% in patients taking placebo. Elevated transaminases leading to discontinuation of study treatment occurred in 0.4% of patients taking simvastatin and 0.2% of patients taking placebo. The majority of elevated transaminases leading to treatment discontinuation occurred within in the first year.
Adverse Reactions in Pediatric Patients with Heterozygous Familial Hypercholesterolemia
In a 48-week clinical study in pediatric patients 10 years of age and older (43% female, 97.7% Caucasians, 1.7% Hispanics, 0.6% Multiracial) with HeFH (n=175), treated with placebo or simvastatin (10 - 40 mg daily), the most common adverse reactions were upper respiratory infection, headache, abdominal pain, and nausea [see USE IN SPECIFIC POPULATIONS (8.4) AND CLINICAL STUDIES (14)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of simvastatin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as whole
fever, chills, malaise, asthenia
Blood and Lymphatic System Disorders
anemia, thrombocytopenia, leukopenia, hemolytic anemia, positive ANA, ESR increase, eosinophilia
Gastrointestinal Disorders
pancreatitis, vomiting
Hepatic and Pancreatic Disorders****
hepatitis/jaundice, fatal and non-fatal hepatic failure
Immune System Disorders****
hypersensitivity syndrome including: anaphylaxis, angioedema, lupus erythematous-like syndrome, dermatomyositis, vasculitis
Musculoskeletal and Connective Tissue Disorders
muscle cramps, immune-mediated necrotizing myopathy, polymyalgia rheumatica, arthritis
Nervous System Disorders****
dizziness, depression, paresthesia, peripheral neuropathy. Rare reports of cognitive impairment (e.g., memory loss, forgetfulness, amnesia, memory impairment, confusion) associated with statin use. Cognitive impairment was generally nonserious, and reversible upon statin discontinuation, with variable times to symptom onset (1 day to years) and symptom resolution (median of 3 weeks). There have been rare reports of new-onset or exacerbation of myasthenia gravis, including ocular myasthenia, and reports of recurrence when the same or a different statin was administered.
Skin and Subcutaneous Tissue Disorders****
pruritus, alopecia, a variety of skin changes (e.g., nodules, discoloration, dryness of skin/mucous membranes, changes to hair/nails), purpura, lichen planus, urticaria, photosensitivity, flushing, toxic epidermal necrolysis, erythema multiforme, including Stevens-Johnson syndrome
Respiratory and Thoracic****
interstitial lung disease, dyspnea
Reproductive System Disorders****
erectile dysfunction
Most common adverse reactions (incidence ≥5%) are: upper respiratory infection, headache, abdominal pain, constipation, and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contactLupin Pharmaceuticals, Inc. at1-800-399-2561 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DESCRIPTION SECTION
11 DESCRIPTION
Simvastatin is a prodrug of 3-hydoroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitor that is derived synthetically from a fermentation product of Aspergillus terreus.
Simvastatin is butanoic acid, 2,2-dimethyl-,1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-(tetrahydro-4-hydroxy-6-oxo-2H-pyran-2-yl)-ethyl]-1-naphthalenyl ester, [1S-[1α,3α,7β,8β(2S*,4S*),-8aβ]]. The empirical formula of simvastatin is C25H38O5 and its molecular weight is 418.57. Its structural formula is:
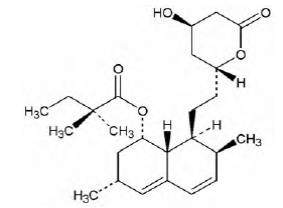
Simvastatin is a white to off-white, nonhygroscopic, crystalline powder that is practically insoluble in water, and freely soluble in chloroform, methanol and ethanol.
Simvastatin tablets USP are available for oral administration in strength of 5 mg, 10 mg, 20 mg, 40 mg or 80 mg. Each tablet contains following inactive ingredients: ascorbic acid, citric acid, hydroxy propyl cellulose, hypromellose, iron oxides, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinised starch, talc and titanium dioxide. Butylated hydroxyanisole is added as a preservative.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Myopathy and Rhabdomyolysis
Advise patients that simvastatin may cause myopathy and rhabdomyolysis. Inform patients taking an 80 mg daily dose of simvastatin that they are at an increased risk. Inform patients that the risk is also increased when taking certain types of medication or consuming grapefruit juice and they should discuss all medication, both prescription and over the counter, with their healthcare provider. Instruct patients to inform other healthcare providers prescribing a new medication or increasing the dose of an existing medication that they are taking simvastatin. Instruct patients to promptly report any unexplained muscle pain, tenderness or weakness particularly if accompanied by malaise or fever [see CONTRAINDICATIONS (4), WARNINGS AND PRECAUTIONS (5.1), AND DRUG INTERACTIONS (7.1)].
Hepatic Dysfunction
Inform patients that simvastatin may cause liver enzyme elevations and possibly liver failure. Advise patients to promptly report fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice [see WARNINGS AND PRECAUTIONS (5.3)].
Increases in HbA1c and Fasting Serum Glucose Levels
Inform patients that increases in HbA1c and fasting serum glucose levels may occur with simvastatin. Encourage patients to optimize lifestyle measures, including regular exercise, maintaining a healthy body weight, and making healthy food choices [see WARNINGS AND PRECAUTIONS (5.4)].
Pregnancy
Advise pregnant patients and patients who can become pregnant of the potential risk to a fetus. Advise patients to inform their healthcare provider of a known or suspected pregnancy to discuss if simvastatin should be discontinued [see USE IN SPECIFIC POPULATIONS (8.1)].
Lactation
Advise patients that breastfeeding is not recommended during treatment with simvastatin [see USE IN SPECIFIC POPULATIONS (8.2)].
Missed Dose
Instruct patients to take simvastatin tablets only as prescribed. If a dose is missed, it should be taken as soon as possible. Advise patients not to double their next dose.
LUPIN and the
 are
registered trademarks of Lupin Pharmaceuticals, Inc.
are
registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
MADE IN INDIA.
June 2024
SPL PATIENT PACKAGE INSERT SECTION
SPL PATIENT PACKAGE INSERT
PATIENT INFORMATION
Simvastatin (SIM-va-stat-in)
tablets, for oral use
Rx Only
Read this Patient Information before you start taking simvastatin tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What are simvastatin tablets?
Simvastatin tablets are a prescription medicine that contains the cholesterol lowering medicine, simvastatin. Simvastatin tablets are used to lower:
- the risk of death by lowering the risk of heart disease death.
- the risk of heart attacks and strokes.
- the need for certain types of heart and blood vessel procedures to improve blood flow called arterial revascularization in people with known heart, cerebrovascular disease (conditions that affect blood flow and the blood vessels in the brain), peripheral vascular disease (a blood circulation disorder that causes the blood vessels outside of your heart and brain to narrow, block, or spasm), and diabetes, who are at high risk for heart disease problems.
Simvastatin tablets are used along with diet to:
- lower the level of low-density lipoprotein (LDL) cholesterol or "bad" cholesterol in adults with hyperlipidemia (high levels of fat in the blood), and in adults and children 10 years of age and older with heterozygous familial hypercholesterolemia (an inherited condition that causes high levels of LDL).
- treat adults with a type of high cholesterol called primary dysbetalipoproteinemia.
- lower the level of triglycerides (type of fat in the blood) in adults.
Simvastatin tablets are used along with other cholesterol lowering treatments to lower the level of low-density lipoprotein (LDL) in adults with a type of high cholesterol called homozygous familial hypercholesterolemia (an inherited condition that causes high levels of LDL). The safety and effectiveness of simvastatin tablet has not been established in children younger than 10 years of age with heterozygous familial hypercholesterolemia (HeFH) or other types of hyperlipidemia (high levels of fat in the blood).
Do not take simvastatin tablets if you:
- take certain medicines called CYP3A4 inhibitors such as:
о certain antifungal medicines (such as itraconazole, ketoconazole, posaconazole, voriconazole).
о certain antibiotics (including erythromycin, clarithromycin).
о HIV protease inhibitors (such as indinavir, nelfinavir, ritonavir, and darunavir / ritonavir) and cobicistat containing products such as (elvitegravir / cobicistat / emtricitabine/tenofovir disoproxil fumarate).
о certain hepatitis C virus protease inhibitors (such as boceprevir or telaprevir).
о the antidepressant nefazodone.
- take medicines called cyclosporine, danazol, or gemfibrozil.
- have liver problems.
- are allergic to simvastatin or any of the ingredients in simvastatin tablets. See the end of this Patient Information leaflet for a complete list of ingredients in simvastatin tablets.
Ask your healthcare provider or pharmacist if you are not sure if your medicine is listed above.
Before you take simvastatin tablets, tell your healthcare provider about all of your medical conditions, including if you:
- have unexplained muscle aches or weakness.
- have or have had myasthenia gravis (a disease causing general muscle weakness including in some cases muscles used for breathing), ocular myasthenia (a disease causing eye muscle weakness).
- have kidney problems.
- have liver problems or drink more than 2 glasses of alcohol daily.
- have thyroid problems.
- are 65 years of age or older.
- are of Chinese descent.
- are pregnant or plan to become pregnant. If you become pregnant while taking simvastatin tablets, call
- your healthcare provider right away to discuss stopping simvastatin tablets.
- are breastfeeding or plan to breastfeed. It is not known if simvastatin passes into your breast milk.Do notbreastfeed while taking simvastatin tablets.
**Tell your healthcare provider about all the medicines you take,**including prescription and over-the-counter medicines, vitamins, and herbal supplements. Talk to your healthcare provider before you start taking any new medicines.
Tell your healthcare provider who prescribes simvastatin tablets if another healthcare provider increases the dose of another medicine you are taking.
Simvastatin tablets may affect the way other medicines work, and other medicines may affect how simvastatin tablets works. Especially tell your healthcare provider if you take:
- digoxin (a drug used to treat irregular heartbeat).
- coumarin anticoagulants (drugs that prevent blood clots, such as warfarin).
Taking simvastatin tablets with certain substances can also increase the risk of muscle problems. Especially tell your healthcare provider if you take:
- amiodarone or dronedarone (medicines used to treat an irregular heartbeat).
- verapamil, diltiazem, amlodipine, or ranolazine (medicines used to treat high blood pressure, chest pain associated with heart disease, or other heart conditions).
- lomitapide (a medicine used to treat a serious and rare genetic cholesterol condition).
- daptomycin (a drug used to treat complicated skin and bloodstream infections).
- large doses of niacin or nicotinic acid, especially if you are of Chinese descent.
- fibric acid derivatives (such as fenofibrate).
- colchicine (a medicine used to treat gout).
- grapefruit juice.
Ask your healthcare provider or pharmacist for a list of medicines if you are not sure. Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take simvastatin tablets?
- Take simvastatin tablets exactly as your healthcare provider tells you to take it.
- Do not change your dose or stop taking simvastatin tablets without talking to your healthcare provider.
- Take simvastatin tablets 1 time each day in the evening.
- If you miss a dose, take it as soon as you remember. If you do not remember until it is time for your next dose, skip the missed dose and go back to your regular schedule. Do not take 2 doses of simvastatin tablets at the same time. Talk with your healthcare provider if you have questions about a missed dose.
- While taking simvastatin tablets, continue to follow your cholesterol-lowering diet and to exercise as your healthcare provider told you to.
- Your healthcare provider may do blood tests to check your cholesterol while you take simvastatin tablets. Your healthcare provider may change your dose of simvastatin tablets if needed.
- In case of overdose, get medical help or contact a live Poison Center expert right away at 1-800-222-1222. Advice is also available online at poisonhelp.org
What are the possible side effects of simvastatin tablets?
Simvastatin tablets may cause serious side effects including:
*Muscle pain, tenderness, and weakness (myopathy). Muscle problems, including muscle breakdown, can be serious in some people and rarely cause kidney damage that can lead to death.
Tell your healthcare provider right away if:
оyou have unexplained muscle pain, tenderness, or weakness, especially if you have a fever or feel more tired than usual, while you take simvastatin tablets.
о you have muscle problems that do not go away even after your healthcare provider has advised you to stop taking simvastatin tablets. Your healthcare provider may do further tests to diagnose the cause of your muscle problems.
Your chances of getting muscle problems are higher if you:
о are taking certain other medicines while you take simvastatin tablets.
о are 65 years of age or older.
о are female.
о have thyroid problems (hypothyroidism) that are not controlled.
о have kidney problems.
о are taking higher doses of simvastatin tablets.
о are Chinese.
***Liver problems.**Your healthcare provider should do blood tests to check your liver before you start taking simvastatin tablets and if you have any symptoms of liver problems while you take simvastatin tablets. Call your healthcare provider right away if you have the following symptoms of liver problems: * feeling tired or weak * loss of appetite * right-sided upper belly pain * dark urine * yellowing of your skin or the whites of your eyes
***Increase in blood sugar (glucose) levels).**Simvastatin tablets may cause an increase in your blood sugar levels.
The most common side effects of simvastatin tablets include:
- upper respiratory infection
- headache
- stomach (abdominal) pain
- constipation
- nausea
Tell your healthcare provider if you have any side effect that bothers you or does not go away. These are not all the possible side effects of simvastatin tablets. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store simvastatin tablets?
- Store simvastatin tablets between 41°F to 86°F (5°C to 30°C).
Keep simvastatin tablets and all medicines out of the reach of children.
General information about safe and effective use of simvastatin tablets.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use simvastatin tablets for a condition for which it was not prescribed. Do not give simvastatin tablets to other people, even if they have the same symptoms that you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about simvastatin tablets that is written for health professionals.
What are the ingredients in simvastatin tablets?
Active ingredient: simvastatin.
Inactive ingredients: ascorbic acid, citric acid, hydroxy propyl cellulose, hypromellose, iron oxides, lactose monohydrate, magnesium stearate, microcrystalline cellulose, pregelatinised starch, talc and titanium dioxide. Butylated hydroxyanisole is added as a preservative.
LUPIN and the
 are
registered trademarks of Lupin Pharmaceuticals, Inc.
are
registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: June 2024 ID#: 275725
