Trientine hydrochloride
Trientine Hydrochloride Capsules, USP
Approved
Approval ID
e7950a50-7528-4458-8b3a-34bb1662f83b
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Oct 17, 2022
Manufacturers
FDA
Zydus Lifesciences Limited
DUNS: 918596198
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Trientine hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code70771-1438
Application NumberANDA211554
Product Classification
M
Marketing Category
C73584
G
Generic Name
Trientine hydrochloride
Product Specifications
Route of AdministrationORAL
Effective DateOctober 17, 2022
FDA Product Classification
INGREDIENTS (11)
FERRIC OXIDE REDInactive
Code: 1K09F3G675
Classification: IACT
TRIENTINE HYDROCHLORIDEActive
Quantity: 250 mg in 1 1
Code: HC3NX54582
Classification: ACTIB
FERRIC OXIDE YELLOWInactive
Code: EX438O2MRT
Classification: IACT
FERROSOFERRIC OXIDEInactive
Code: XM0M87F357
Classification: IACT
GELATINInactive
Code: 2G86QN327L
Classification: IACT
POTASSIUM HYDROXIDEInactive
Code: WZH3C48M4T
Classification: IACT
PROPYLENE GLYCOLInactive
Code: 6DC9Q167V3
Classification: IACT
SHELLACInactive
Code: 46N107B71O
Classification: IACT
STEARIC ACIDInactive
Code: 4ELV7Z65AP
Classification: IACT
TITANIUM DIOXIDEInactive
Code: 15FIX9V2JP
Classification: IACT
WATERInactive
Code: 059QF0KO0R
Classification: IACT
Drug Labeling Information
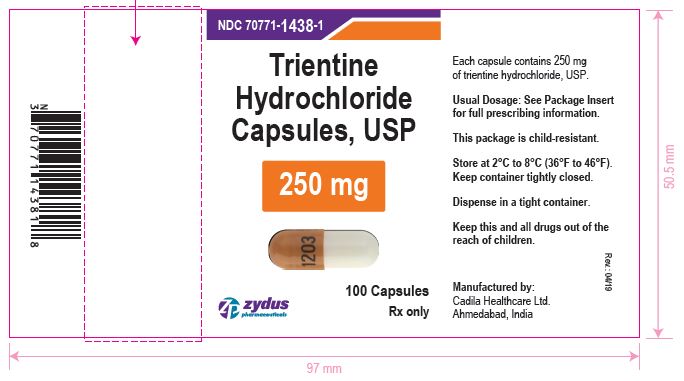
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 4/29/2019
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC 70771-1438-1
Trientine Hydrochloride Capsules, USP 250 mg
100 Capsules
Rx only
SPL UNCLASSIFIED SECTION
LOINC: 42229-5Updated: 8/4/2020
