Risedronate Sodium
RISEDRONATE SODIUM DELAYED-RELEASE TABLETS
Approved
Approval ID
37c8bed0-8cfe-445d-b506-bd89c8fae1a9
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Sep 27, 2023
Manufacturers
FDA
Zydus Lifesciences Limited
DUNS: 918596198
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Risedronate Sodium
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code70771-1436
Application NumberANDA203822
Product Classification
M
Marketing Category
C73584
G
Generic Name
Risedronate Sodium
Product Specifications
Route of AdministrationORAL
Effective DateSeptember 27, 2023
FDA Product Classification
INGREDIENTS (11)
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
EDETATE DISODIUMInactive
Code: 7FLD91C86K
Classification: IACT
FERRIC OXIDE YELLOWInactive
Code: EX438O2MRT
Classification: IACT
STEARIC ACIDInactive
Code: 4ELV7Z65AP
Classification: IACT
POLYSORBATE 80Inactive
Code: 6OZP39ZG8H
Classification: IACT
CELLULOSE, MICROCRYSTALLINEInactive
Code: OP1R32D61U
Classification: IACT
SODIUM STARCH GLYCOLATE TYPE A POTATOInactive
Code: 5856J3G2A2
Classification: IACT
METHACRYLIC ACID AND ETHYL ACRYLATE COPOLYMERInactive
Code: NX76LV5T8J
Classification: IACT
TALCInactive
Code: 7SEV7J4R1U
Classification: IACT
TRIETHYL CITRATEInactive
Code: 8Z96QXD6UM
Classification: IACT
RISEDRONATE SODIUM ANHYDROUSActive
Quantity: 35 mg in 1 1
Code: OFG5EXG60L
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 9/27/2023
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
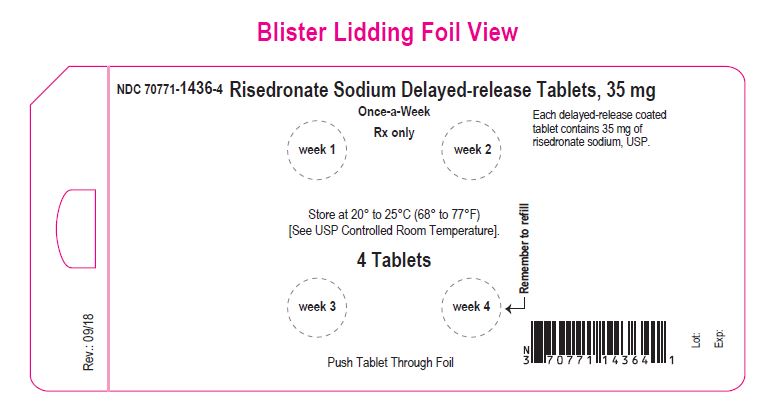
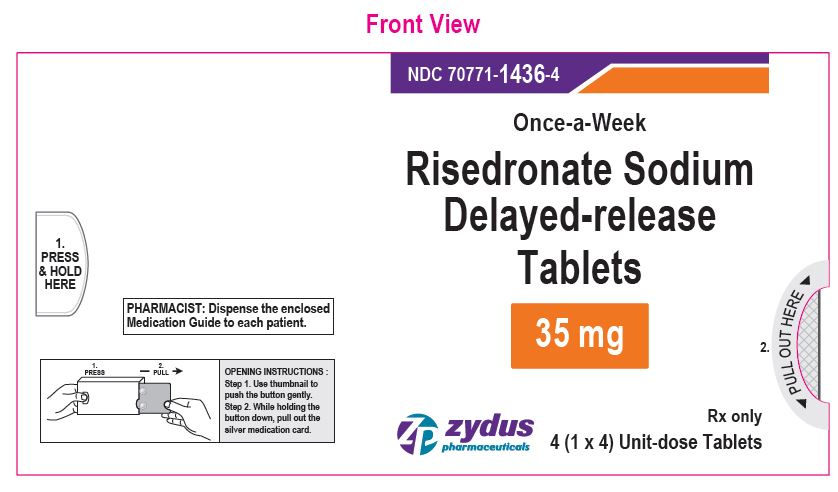
NDC 70771-1436-4 in blister-carton of 4 (1 x 4) Unit-dose Tablets
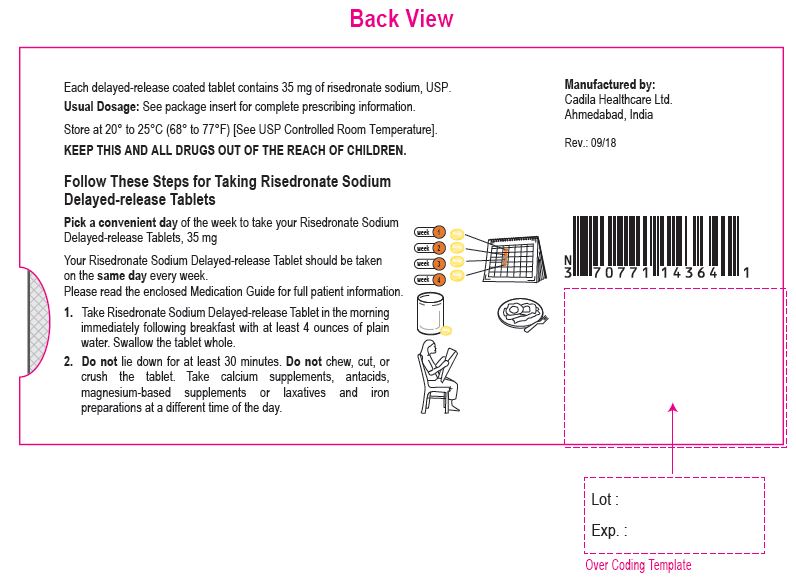
Risedronate Sodium Delayed-release Tablets, 35 mg
Rx only
4 Tablets