Crest Pro-Health
Crest PRO-HEALTH GUM CARE
d721e2cb-8f67-3d07-e053-2995a90ae65a
HUMAN OTC DRUG LABEL
Jul 31, 2025
The Procter & Gamble Manufacturing Company
DUNS: 004238200
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Cetylpyridinium chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Drug Labeling Information
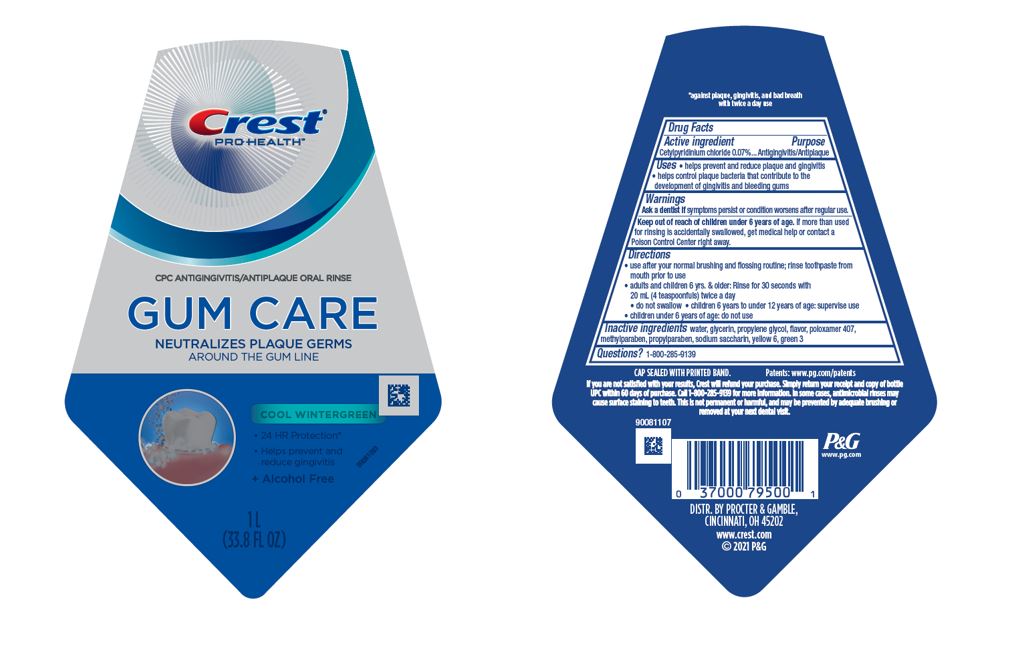
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 1 L Bottle Label
Crest®
Pro Health
CPC ANTIGINGIVITIS/ANTIPLAQUE ORAL RINSE
GUM CARE
NEUTRALIZES PLAQUE GERMS
AROUND THE GUM LINE
COOL WINTERGREEN
• 24 HR Protection*
• Helps prevent and reduce gingivitis
+ Alcohol Free
1 L
(33.8 FL OZ)
INDICATIONS & USAGE SECTION
Uses
- helps prevent and reduce plaque and gingivitis
- helps control plaque bacteria that contribute to the development of gingivitis and bleeding gums
SPL UNCLASSIFIED SECTION
DISTR. BY PROCTER & GAMBLE,
CINCINNATI, OH 45202
OTC - ACTIVE INGREDIENT SECTION
Active ingredient
Cetylpyridinium chloride 0.07%
OTC - PURPOSE SECTION
Purpose
Antigingivitis/antiplaque
WARNINGS SECTION
Warnings
Ask a dentist ifsymptoms persist or condition worsens after regular use.
**Keep out of reach of children under 6 years of age.**If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- use after your normal brushing and flossing routine; rinse toothpaste from mouth prior to use
- adults and children 6 yrs. & older: Rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- children under 6 years of age: do not use
INACTIVE INGREDIENT SECTION
Inactive ingredients
water, glycerin, propylene glycol, flavor, poloxamer 407, methylparaben, propylparaben, sodium saccharin, yellow 6, green 3
OTC - QUESTIONS SECTION
Questions?
1–800–285–9139
