Lovastatin
LOVASTATIN TABLETS USP10 mg, 20 mg and 40 mgRx only
df7ddf4f-d569-431e-81f1-9129d7043150
HUMAN PRESCRIPTION DRUG LABEL
Nov 30, 2023
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
lovastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
lovastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
lovastatin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (9)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
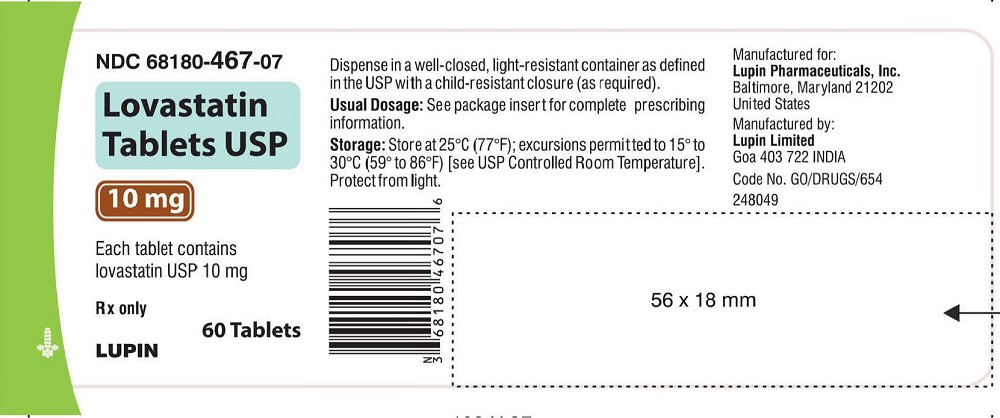
NDC 68180-467-07
LOVASTATIN TABLETS USP
10 mg
Rx only
Bottle of 60 Tablets
NDC 68180-468-07
LOVASTATIN TABLETS USP
20 mg
Rx only
Bottle of 60 Tablets

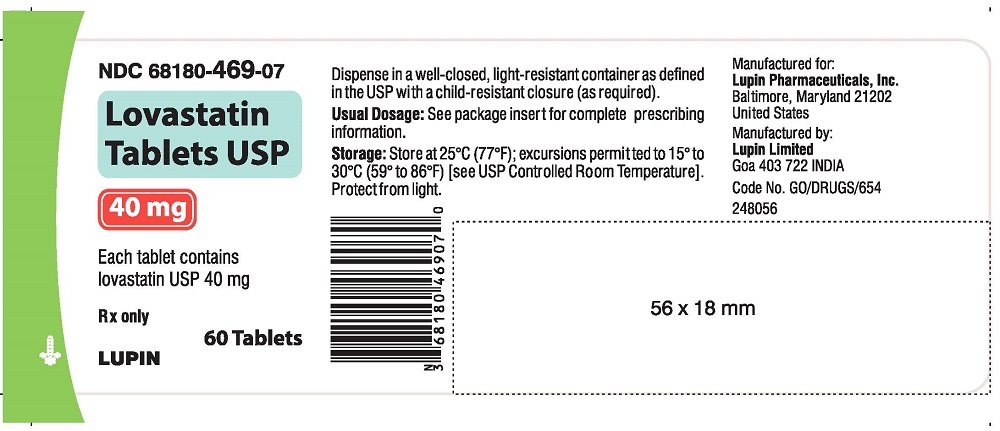
NDC 68180-469-07
LOVASTATIN TABLETS USP
40 mg
Rx only
Bottle of 60 Tablets