RUFINAMIDE
These highlights do not include all the information needed to use RUFINAMIDE TABLETS, USP safely and effectively. See full prescribing information for RUFINAMIDE TABLETS, USP.RUFINAMIDE tablets, for oral useInitial U.S. Approval: 2008
2c2c8d41-1ae4-453a-b628-954ee0b3be5c
HUMAN PRESCRIPTION DRUG LABEL
Aug 1, 2025
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
rufinamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
rufinamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
rufinamide
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rx Only
NDC 68180-802-06
Rufinamide Tablets, 100 mg
Container Label of 30 Tablets
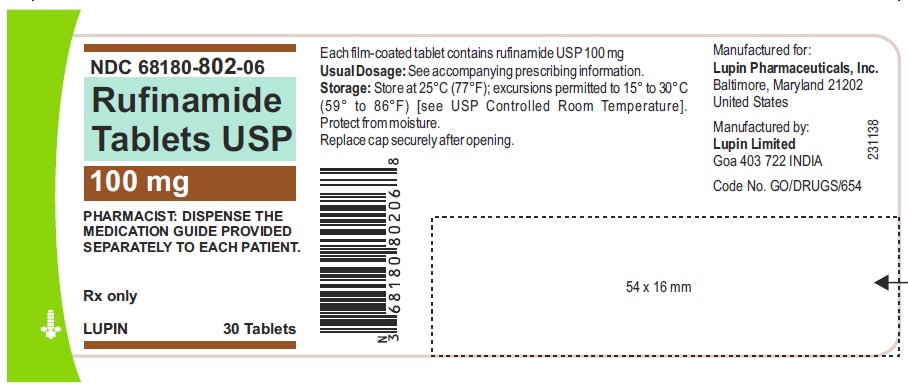
Image a
Rx Only
NDC 68180-803-16
Rufinamide Tablets, 200 mg
Container Label of 120 Tablets
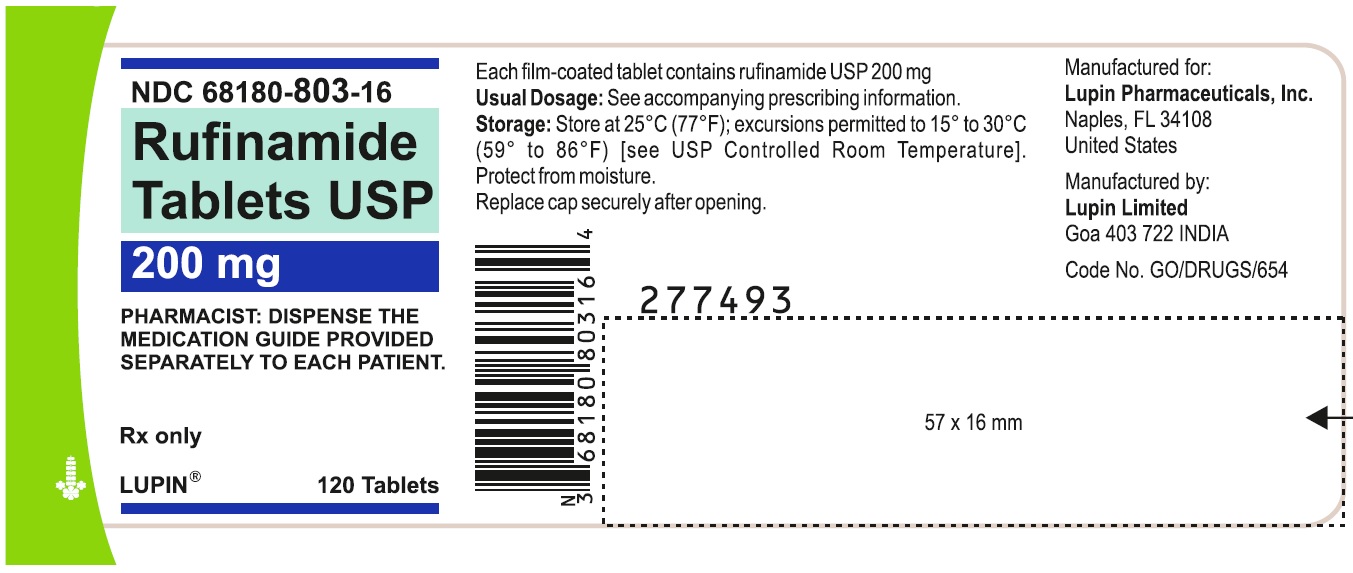
image B
Rx Only
NDC 68180-804-16
Rufinamide Tablets, 400 mg
Container Label of 120 Tablets
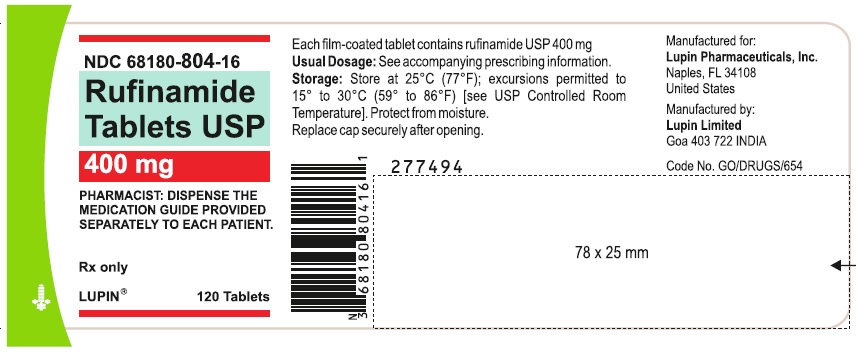
Image C
DESCRIPTION SECTION
11 DESCRIPTION
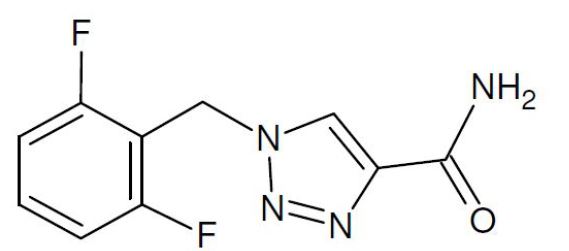
Rufinamide is a triazole derivative structurally unrelated to currently marketed antiepileptic drugs (AEDs). Rufinamide has the chemical name 1-[(2,6-difluorophenyl)methyl]-1H -1,2,3-triazole-4 carboxamide. It has an empirical formula of C10H8F2N4O and a molecular weight of 238.194. The drug substance is a white to off white color powder, crystalline, odorless and slightly bitter tasting neutral powder. Rufinamide is slightly soluble in dimethylsulfoxide, tetrahydrofuran and in methanol, very slightly soluble in alcohol and in acetonitrile, practically insoluble in water.
Rufinamide tablet USP is available for oral administration in film-coated tablets, scored on both sides, containing 100 mg, 200 mg and 400 mg of rufinamide. Inactive ingredients are colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose, iron oxide red, lactose monohydrate, magnesium stearate, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulphate, talc, titanium dioxide.
USP Dissolution Test 2 is used.
SPL MEDGUIDE SECTION
Medication Guide
Rufinamide**(roo-FIN-a-mide)**
** Tablets USP**
Rx only
Read this Medication Guide before you start taking rufinamide tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about rufinamide tablets?
Do not stop taking rufinamide tablets without first talking to your healthcare provider.
Stopping rufinamide tablets suddenly can cause serious problems.
Rufinamide tablets can cause serious side effects, including:
1. Like other antiepileptic drugs, rufinamide tablets may cause suicidal thoughts or actions in a very small number of people, about 1 in 500.
Call a healthcare provider right away if you have any of these symptoms, especially if they are new, worse, or worry you:
- thoughts about suicide or dying
- attempt to commit suicide
- new or worse depression
- new or worse anxiety
- feeling agitated or restless
- panic attacks
- trouble sleeping (insomnia)
- new or worse irritability
- acting aggressive, being angry, or violent
- acting on dangerous impulses
- an extreme increase in activity and talking (mania)
- other unusual changes in behavior or mood
- Suicidal thoughts or actions can be caused by things other than medicines. If you have suicidal thoughts or actions, your healthcare provider may check for other causes.
How can I watch for early symptoms of suicidal thoughts and actions?
- Pay attention to any changes, especially sudden changes, in mood, behaviors, thoughts, or feelings.
- Keep all follow-up visits with your healthcare provider as scheduled.
Call your healthcare provider between visits as needed, especially if you are worried about symptoms.
Do not stop rufinamide tablets without first talking to a healthcare provider.
- Stopping rufinamide tablets suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy can cause seizures that will not stop (status epilepticus).
2. Rufinamide tablets may cause you to feel sleepy, tired, weak, dizzy or have problems with coordination and walking.
What is rufinamide tablet?
Rufinamide tablet is a prescription medicine used with other medicines to treat seizures associated with Lennox-Gastaut Syndrome (LGS) in adults and pediatric patients 1 year of age and older.
It is not known if rufinamide tablet is safe and effective in the treatment of Lennox-Gastaut Syndrome in pediatric patients under 1 year of age.
Who should not take rufinamide tablets?
Do not take rufinamide tablets if you have a genetic condition called familial short QT syndrome, a problem that affects the electrical system of the heart.
**What should I tell my healthcare provider before taking rufinamide tablets? **
Before you take rufinamide tablets, tell your healthcare provider if you:
- have heart problems
- have liver problems
- have any other medical problems
- have or have had suicidal thoughts or actions, depression or mood problems
- are pregnant or plan to become pregnant. It is not known if rufinamide tablets can harm your unborn baby. Tell your healthcare provider right away if you become pregnant while taking rufinamide tablets. You and your healthcare provider will decide if you should take rufinamide tablets while you are pregnant.
- Rufinamide tablets may make certain types of birth control less effective. Talk to your healthcare provider about the best birth control methods for you while you take rufinamide tablets.
- If you become pregnant while taking rufinamide tablets, talk to your healthcare provider about registering with the North American Antiepileptic Drug Pregnancy Registry. You can enroll in this registry by calling 1-888-233-2334. The purpose of this registry is to collect information about the safety of antiepileptic medicines during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if rufinamide will pass into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you take rufinamide.
**Tell your healthcare provider about all the medicines you take,**including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking rufinamide tablets with certain other medicines can cause side effects or affect how well they work. Do not start or stop other medicines without talking to your healthcare provider.
Know the medicines you take. Keep a list of them and show it to your healthcare provider and pharmacist each time you get a new medicine.
How should I take rufinamide tablets?
- Take rufinamide tablets exactly as your healthcare provider tells you. Your healthcare provider will tell you how much rufinamide tablets to take.
- Your healthcare provider may change your dose. Do not change your dose of rufinamide tablets without talking to your healthcare provider.
- Take rufinamide tablets with food.
- Rufinamide tablets can be swallowed whole, cut in half or crushed.
- If you take too much rufinamide tablets, call your local Poison Control Center or get emergency medical help right away.
What should I avoid while taking rufinamide tablets?
- Do not drink alcohol or take other medicines that make you sleepy or dizzy while taking rufinamide tablets until you talk to your healthcare provider. Rufinamide tablets taken with alcohol or medicines that cause sleepiness or dizziness may make your sleepiness or dizziness worse.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how rufinamide tablets affects you. Rufinamide tablets can slow your thinking and motor skills.
What are the possible side effects of rufinamide tablets?
See "What is the most important information I should know about rufinamide tablets?"
Rufinamide tablets may cause serious side effects including:
- Rufinamide tablets can also cause allergic reactions or serious problems which may affect organs and other parts of your body like the liver or blood cells. You may or may not have a rash with these types of reactions.
Call your healthcare provider right away if you have any of the following. Symptoms may include:
- swelling of your face, eyes, lips, or tongue
- trouble swallowing or breathing
- a skin rash
- hives
- fever, swollen glands, or sore throat that do not go away or come and go
- swollen glands
- yellowing of your skin or eyes
- dark urine
- unusual bruising or bleeding
- severe fatigue or weakness
- severe muscle pain
- your seizures happen more often or become worse
Call your healthcare provider right away if you have any of the symptoms listed above.
The most common side effects of rufinamide tablets include:
- headache
- dizziness
- tiredness
- sleepiness
- nausea
- vomiting
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all of the possible side effects of rufinamide tablets. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088.
How should I store rufinamide tablets?
Store rufinamide tablets at 59ºF to 86ºF (15ºC to 30ºC).
Keep rufinamide tablets in a dry place.
Keep rufinamide tablets and all medicines out of the reach of children.
**General Information about the safe and effective use of rufinamide tablets **
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use rufinamide tablets for a condition for which it was not prescribed. Do not give rufinamide tablets to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about rufinamide tablets. If you would like more information, talk with your doctor. You can ask your pharmacist or doctor for information about rufinamide tablets that is written for health professionals.
For more information, go to www.lupinpharmaceuticals.com or call 1-800-399-2561.
What are the ingredients in rufinamide tablets?
Active ingredient: rufinamide
Inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose, iron oxide red, lactose monohydrate, magnesium stearate, polyethylene glycol, polyvinyl alcohol, sodium lauryl sulphate, talc, titanium dioxide.
This Medication Guide has been approved by the U.S. Food and Drug Administration.
The brands listed are trademarks of their respective owners and are not trademarks of Lupin Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Lupin Pharmaceuticals, Inc. or its products.
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
Manufactured by:
Lupin Limited
Goa 403 722
INDIA
Revised: October 2024 ID#: 277507
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Administration Information
- Advise patients to take rufinamide tablets with food[see DOSAGE AND ADMINISTRATION (2.2)] .
Suicidal Thinking and Behavior
Inform patients, their caregivers, and families that antiepileptic drugs increase the risk of suicidal thoughts and behavior, and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers [see WARNINGS AND PRECAUTIONS (5.1)].
Central Nervous System Reactions
Inform patients about the potential for somnolence or dizziness and advise them not to drive or operate machinery until they have gained sufficient experience on rufinamide tablets to gauge whether it adversely affects their mental and/or motor performance [see WARNINGS AND PRECAUTIONS (5.2)].
Multi-Organ Hypersensitivity Reactions
Advise patients to notify their physician if they experience a rash associated with fever [see WARNINGS AND PRECAUTIONS (5.4)].
Drug Interactions
- Inform female patients of childbearing age that the concurrent use of rufinamide tablets with hormonal contraceptives may render this method of contraception less effective. Recommend patients use additional non-hormonal forms of contraception when using rufinamide tablets [see DRUG INTERACTIONS (7.3) and USE IN SPECIFIC POPULATIONS (8.3)] .
- Inform patients that alcohol in combination with rufinamide tablets may cause additive central nervous system effects.
Pregnancy
Advise patients to notify their physician if they become pregnant or intend to become pregnant during therapy. Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. To enroll, patients can call the toll free number 1-888-233-2334 [see USE IN SPECIFIC POPULATIONS (8.1)].
Breast-feeding
Advise patients to notify their physician if they are breast-feeding or intend to breast-feed [see USE IN SPECIFIC POPULATIONS (8.2)].
The brands listed are trademarks of their respective owners and are not trademarks of Lupin Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Lupin Pharmaceuticals, Inc. or its products.
Manufactured for:
LUPIN and the
 are registered trademarks of Lupin Pharmaceuticals, Inc.
are registered trademarks of Lupin Pharmaceuticals, Inc.
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
Manufactured by:
Lupin Limited
Goa 403 722
INDIA
Revised: October 2024 ID#: 277506
