Optiq Balance
OPTIQ RESTORE ADVANCED LIPID COMPLEX MGD RELIEF PRESERVATIVE FREE
e9fbc4b5-716a-4caa-9bab-b4a498181114
HUMAN OTC DRUG LABEL
Sep 20, 2025
Optiq Eyecare LLC
DUNS: 139782805
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Light Mineral Oil, Mineral Oil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
Optiq Eyecare
NDC: 85949-113-10
Optiq
RESTORE
ADVANCED LIPID COMPLEX
MGD RELIEF

INDICATIONS & USAGE SECTION
Uses
- Temporary relief of burning and irritation due to dryness of the eye
- Temporary relief of discomfort due to minor irritations of the eye or to exposure to wind or sun
- As a protectant to prevent further irritation or to relieve dryness of the eye
OTC - ACTIVE INGREDIENT SECTION
Drug Facts
|
Active Ingredients |
Purpose |
|
Light mineral oil (0.6%) |
Emollient |
|
Mineral oil (0.6%) |
Emollient |
WARNINGS SECTION
Warnings
For external use only
Do not use
- if emulsion changes color
- if you are sensitive to any ingredient in this product
When using this product
- Do not touch the tip of container to any surface to avoid contamination
- Replace cap after each use
Stop use and ask a doctor if****you experience any of the following:
- Eye pain
- Changes in vision
- Continued redness or irritation of the eye
- Condition worsens or persists for more than 72 hours
**Keep out of reach of children.**If swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- Shake well before use
- Instill 1 to 2 drop(s) in the affected eye(s) as needed or as directed by your doctor
- See side of carton for directions for use
SPL UNCLASSIFIED SECTION
Other Information
- Temporarily blurred vision is typical upon application
- Drops appear as a milky white emulsion
- Store at 15-25°C (59-77°F)
INACTIVE INGREDIENT SECTION
Inactive Ingredients
Gellan Gum, Glyceryl Monostearate, Polysorbate 80, Hypromellose, Sodium Hyaluronate, Trehalose, Mannitol, Tromethamine and Purified Water.
OTC - QUESTIONS SECTION
Questions?
Call: 1-877-696-7848
hello@optiqeyecare.com
www.optiqeyecare.com
Directions for Use:
1. Hold the bottle just below the cap and twist the cap to open.
2. Before first use, hold the bottle upside down and dispense one drop of
product to discard.
3. Shake the bottle downward to remove residual product (see picture).
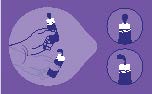
4. Place 1 or 2 drops as needed in the affected eye(s) and blink.
5. After use, shake the bottle downward to remove residual product that may
be left on the tip (picture above).
6. Replace cap after use.
Tamper Evident
For your protection this bottle has a tamper evident ring around the bottom of the cap. Do not use if this ring is damaged or missing at the time of purchase.
Marketed and Distributed by:
Optiq Eyecare
PO Box 32
Paramus, NJ 07652
