Minoxidil
Minoxidil
665cba3d-5f26-468a-a047-188a413fbede
HUMAN PRESCRIPTION DRUG LABEL
Aug 30, 2023
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Minoxidil
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Minoxidil tablets contain minoxidil, an antihypertensive peripheral vasodilator. Minoxidil occurs as a white to off-white, odorless, crystalline solid that is soluble in water to the extent of approximately 2 mg/mL, is readily soluble in propylene glycol or ethanol, and is almost insoluble in acetone, chloroform or ethyl acetate. The chemical name for minoxidil is 2,4-pyrimidinediamine, 6-(1-piperidinyl)-, 3-oxide. The structural formula is represented at right:
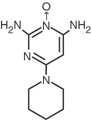
- C 9H 15N 50 MW 209.25
Minoxidil tablets for oral administration contain either 2.5 mg or 10 mg of minoxidil. Inactive ingredients include colloidal silicon dioxide, corn starch, lactose anhydrous, magnesium stearate and microcrystalline cellulose.
