FESOTERODINE FUMARATE
These highlights do not include all the information needed to use FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS safely and effectively. See full prescribing information for FESOTERODINE FUMARATE EXTENDED-RELEASE TABLETS. FESOTERODINE FUMARATE extended-release tablets, for oral use Initial U.S. Approval: 2008
dc8f6158-3c2b-4ec0-a951-e04468b83bb1
HUMAN PRESCRIPTION DRUG LABEL
Aug 30, 2023
Aurobindo Pharma Limited
DUNS: 650082092
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
FESOTERODINE FUMARATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
FESOTERODINE FUMARATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (13)
Drug Labeling Information
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Recommended Dosage for Adult Patients With OAB
The recommended starting dosage of fesoterodine fumarate extended-release tablets in adults is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of fesoterodine fumarate extended-release tablets 8 mg once daily. For administration instructions, see Dosage and Administration (2.6).
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.3 Recommended Dosage in Adult Patients With Renal Impairment
The recommended dosage of fesoterodine fumarate extended-release tablets in adult patients with renal impairment is described in Table 1 [see Use in Specific Populations (8.6)]. For administration instructions, see Dosage and Administration (2.6).
Table 1: Fesoterodine Fumarate Extended-Release Tablets Recommended Dose in Adult Patients With Renal Impairment (Administered Orally Once Daily)|
1 Calculate CLcr using the Cockcroft-Gault formula | |
|
Estimated Creatinine Clearance****1 |
Recommended Dose |
|
CLcr 30 to 89 mL/min |
8 mg |
|
CLcr 15 to 29 mL/min |
4 mg |
|
CLcr <15 mL/min |
4 mg |
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.5 Fesoterodine Fumarate Extended-Release Tablets Dosage Modifications Due
to Strong CYP3A4 Inhibitors
Adult Patients with OAB
The maximum recommended dosage is fesoterodine fumarate extended-release tablets 4 mg orally once daily in adult patients taking strong CYP3A4 inhibitors [see Drug Interactions (7.2) and Clinical Pharmacology (12.3)]. For administration instructions, see Dosage and Administration (2.6).
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
2.6 Administration Instructions
Swallow fesoterodine fumarate extended-release tablets whole with liquid. Do not chew, divide, or crush. Take with or without food [see Clinical Pharmacology (12.3)].
- OAB in Adults: The recommended starting dosage is 4 mg orally once daily. Based upon individual response and tolerability, increase to the maximum dosage of 8 mg once daily. (2.1)
- Adult Patients with Renal Impairment: Refer to the full prescribing information for recommended dosage. (2.3)
- Dosage Modifications Due to Strong CYP3A4 Inhibitors: Refer to the full prescribing information for recommended dosage. (2.5)
- Administration: Swallow whole with liquid. Do not chew, divide, or crush. Take with or without food. (2.6)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
There are no available data with the use of fesoterodine fumarate extended- release tablets in pregnant women and adolescents to evaluate for a drug- associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. In animal reproduction studies, oral administration of fesoterodine to pregnant mice and rabbits during organogenesis resulted in fetotoxicity at maternal exposures that were 6 and 3 times respectively the maximum recommended human dose (MRHD) of 8 mg/day, based on AUC (see Data). The background risk of major birth defects and miscarriage for the indicated population are unknown. However, in the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Animal Data
No dose-related teratogenicity was observed in reproduction studies performed in mice and rabbits. In mice at 6 to 27 times the expected exposure at the maximum recommended human dose (MRHD) of 8 mg based on AUC (75 mg/kg/day, oral), increased resorptions and decreased live fetuses were observed. One fetus with cleft palate was observed at each dose (15, 45, and 75 mg/kg/day), at an incidence within the background historical range. In rabbits treated at 3 to 11 times the MRHD (27 mg/kg/day, oral), incompletely ossified sternebrae (retardation of bone development) and reduced survival were observed in fetuses. In rabbits at 9 to 11 times the MRHD (4.5 mg/kg/day, subcutaneous), maternal toxicity and incompletely ossified sternebrae were observed in fetuses (at an incidence within the background historical range). In rabbits at 3 times the MRHD (1.5 mg/kg/day, subcutaneous), decreased maternal food consumption in the absence of any fetal effects was observed. Oral administration of 30 mg/kg/day fesoterodine to mice in a pre- and post-natal development study resulted in decreased body weight of the dams and delayed ear opening of the pups. No effects were noted on mating and reproduction of the F1 dams or on the F2 offspring.
8.2 Lactation
Risk Summary
There is no information on the presence of fesoterodine in human milk, the effects on the breastfed child, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for fesoterodine fumarate extended-release tablets and any potential adverse effects on the breastfed child from fesoterodine fumarate extended-release tablets or from the underlying maternal condition.
8.4 Pediatric Use
The safety and effectiveness of fesoterodine fumarate extended-release tablets have not been established in pediatric patients younger than 6 years of age or weighing 25 kg or less.
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.5 Geriatric Use
No dose adjustment is recommended for the elderly. The pharmacokinetics of fesoterodine are not significantly influenced by age.
Of the 1,567 patients who received fesoterodine fumarate extended-release tablets 4 mg or 8 mg orally once daily in Phase 2 and 3, placebo-controlled, efficacy and safety studies for OAB, 515 (33%) were 65 years of age or older, and 140 (9%) were 75 years of age or older. No overall difference in effectiveness was observed between patients younger than 65 years of age and those 65 years of age or older in these studies. However, the incidence of antimuscarinic adverse reactions, including dry mouth, constipation, dyspepsia, increase in residual urine, dizziness (8 mg only) and urinary tract infection, was higher in patients 75 years of age and older as compared to younger patients [see Clinical Studies (14.1) and Adverse Reactions (6)].
8.6 Renal Impairment
In adult patients with severe renal impairment (CLCR <30 mL/min), Cmax and AUC are increased 2- and 2.3-fold, respectively. Doses of fesoterodine fumarate extended-release tablets greater than 4 mg are not recommended in adult patients with severe renal impairment. In patients with mild or moderate renal impairment (CLCR ranging from 30 to 80 mL/min), Cmax and AUC of the active metabolite are increased up to 1.5- and 1.8-fold, respectively, as compared to healthy subjects. No dose adjustment is recommended in patients with mild or moderate renal impairment [see Clinical Pharmacology (12.3) and Dosage and Administration (2.3)].
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
8.7 Hepatic Impairment
Patients with severe hepatic impairment (Child-Pugh C) have not been studied; therefore, fesoterodine fumarate extended-release tablets are not recommended for use in these patients. In patients with moderate (Child-Pugh B) hepatic impairment, Cmax and AUC of the active metabolite are increased 1.4- and 2.1-fold, respectively, as compared to healthy subjects. No dose adjustment is recommended in patients with mild or moderate hepatic impairment [see Clinical Pharmacology (12.3)].
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
No evidence of drug-related carcinogenicity was found in 24-month studies with oral administration to mice and rats. The highest tolerated doses in mice (females 45 to 60 mg/kg/day, males 30 to 45 mg/kg/day) correspond to 11 to 19 times (females) and 4 to 9 times (males) the estimated human AUC values reached with fesoterodine 8 mg, which is the Maximum Recommended Human Dose (MRHD). In rats, the highest tolerated dose (45 to 60 mg/kg/day) corresponds to 3 to 8 times (females) and 3 to 14 times (males) the estimated human AUC at the MRHD.
Mutagenesis
Fesoterodine was not mutagenic or genotoxic in vitro (Ames tests, chromosome aberration tests) or in vivo (mouse micronucleus test).
Impairment of Fertility
Fesoterodine had no effect on male reproductive function or fertility at doses up to 45 mg/kg/day in mice. At 45 mg/kg/day, a lower number of corpora lutea, implantation sites and viable fetuses was observed in female mice administered fesoterodine for 2-weeks prior to mating and continuing through day 7 of gestation. The maternal No-Observed-Effect Level (NOEL) and the NOEL for effects on reproduction and early embryonic development were both 15 mg/kg/day. At the NOEL, the systemic exposure, based on AUC, was 0.6 to 1.5 times higher in mice than in humans at the MRHD, whereas based on peak plasma concentrations, the exposure in mice was 5 to 9 times higher.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Adult Overactive Bladder
The efficacy of fesoterodine fumarate extended-release tablets was evaluated in two, Phase 3, randomized, double-blind, placebo-controlled, 12-week studies for the treatment of overactive bladder with symptoms of urge urinary incontinence, urgency, and urinary frequency. Entry criteria required that patients have symptoms of overactive bladder for ≥6-months duration, at least 8 micturitions per day, and at least 6 urinary urgency episodes or 3 urge incontinence episodes per 3-day diary period. Patients were randomized to a fixed dose of fesoterodine fumarate extended-release tablets 4 or 8 mg/day or placebo. In one of these studies, 290 patients were randomized to an active control arm (an oral antimuscarinic agent). For the combined studies, a total of 554 patients received placebo, 554 patients received fesoterodine fumarate extended-release tablets 4 mg/day, and 566 patients received fesoterodine fumarate extended-release tablets 8 mg/day. The majority of patients were Caucasian (91%) and female (79%) with a mean age of 58 years (range 19 to 91 years).
The primary efficacy endpoints were the mean change in the number of urge urinary incontinence episodes per 24 hours and the mean change in the number of micturitions (frequency) per 24 hours. An important secondary endpoint was the mean change in the voided volume per micturition.
Results for the primary endpoints and for mean change in voided volume per micturition from the two 12-week clinical studies of fesoterodine fumarate extended-release tablets are reported in Table 10.
Table 10: Mean Baseline and Change From Baseline to Week 12 for Urge Urinary Incontinence Episodes, Number of Micturitions, and Volume Voided per Micturition
|
Study 1 |
Study 2 | |||||
|
Parameter |
Placebo |
Fesoterodine fumarate extended-release tablets |
Fesoterodine fumarate extended-release tablets |
Placebo |
Fesoterodine fumarate extended-release tablets |
Fesoterodine fumarate extended-release tablets |
|
Number of urge incontinence episodes per 24 hoursa | ||||||
|
Baseline |
3.7 |
3.8 |
3.7 |
3.7 |
3.9 |
3.9 |
|
Change from baseline |
-1.2 |
-2.06 |
-2.27 |
-1 |
-1.77 |
-2.42 |
|
p-value vs. placebo |
|
0.001 |
<0.001 |
|
<0.003 |
<0.001 |
|
Number of micturitions per 24 hours | ||||||
|
Baseline |
12 |
11.6 |
11.9 |
12.2 |
12.9 |
12 |
|
Change from baseline |
-1.02 |
-1.74 |
-1.94 |
-1.02 |
-1.86 |
-1.94 |
|
p-value vs. placebo |
|
<0.001 |
<0.001 |
|
0.032 |
<0.001 |
|
Voided volume per micturition (mL) | ||||||
|
Baseline |
150 |
160 |
154 |
159 |
152 |
156 |
|
Change from baseline |
10 |
27 |
33 |
8 |
17 |
33 |
|
p-value vs. placebo |
|
<0.001 |
<0.001 |
|
0.15 |
<0.001 |
|
vs. = versus |
Figures 1 to 4: The following figures show change from baseline over time in number of micturitions and urge urinary incontinence episodes per 24 h in the two studies.
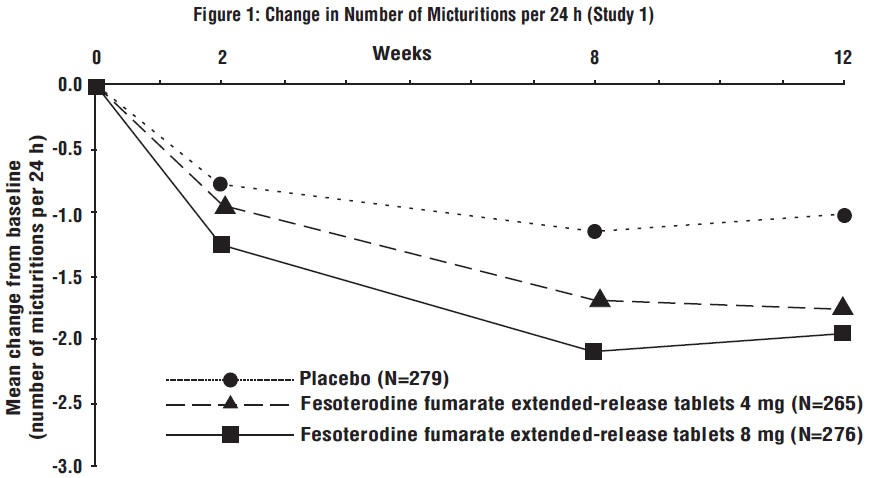
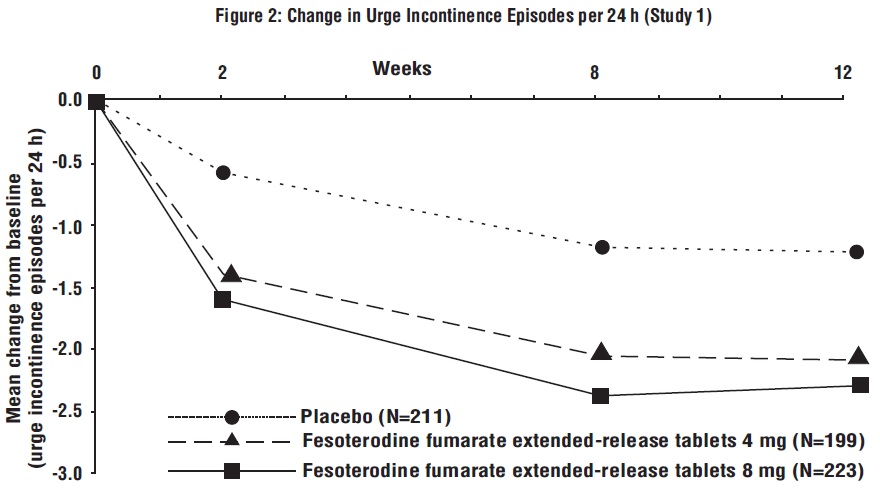
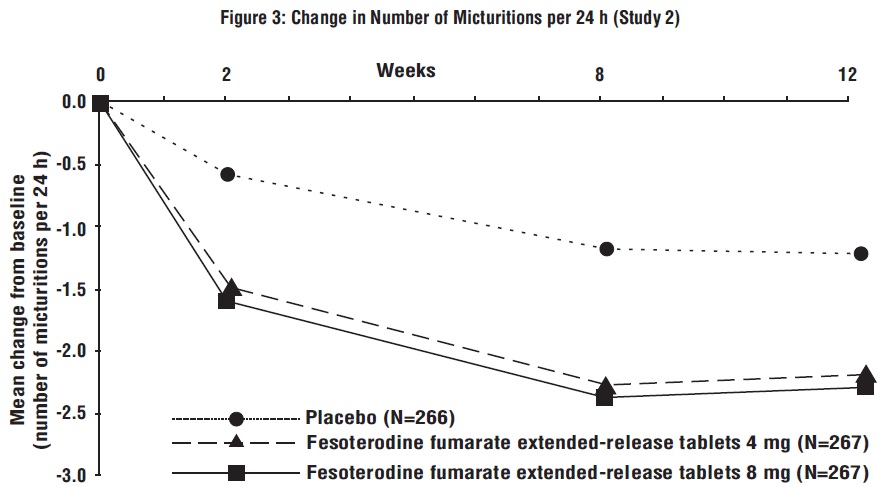
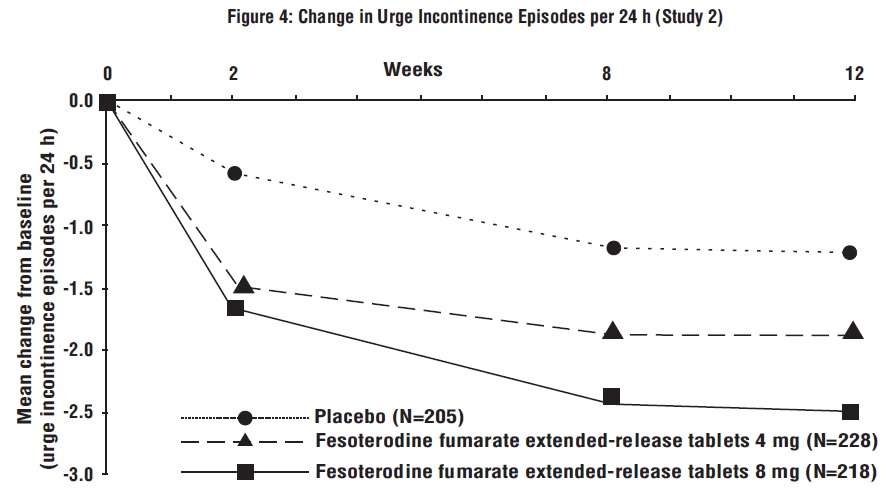
A reduction in number of urge urinary incontinence episodes per 24 hours was observed for both doses as compared to placebo as early as two weeks after starting fesoterodine fumarate extended-release tablets therapy.
Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
SPL PATIENT PACKAGE INSERT SECTION
|
Patient Information |
|
Read the Patient Information that comes with fesoterodine fumarate extended- release tablets before you start taking them and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical condition or your treatment. |
|
What are****fesoterodine fumarate extended-release tablets? Fesoterodine fumarate extended-release tablets are a prescription medicine used:
It is not known if fesoterodine fumarate extended-release tablets are safe and effective in children younger than 6 years of age or with a body weight 55 pounds (25 kg) or less. |
|
Who should not take fesoterodine fumarate extended-release tablets?
|
|
Before you take fesoterodine fumarate extended-release tablets, tell your healthcare provider about all your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal products. Fesoterodine fumarate extended**-release tablets may affect the way other medicines work, and other medicines may affect how fesoterodine fumarate extended-**release tablets work. Especially tell your healthcare provider if you are taking antimuscarinic, antibiotics, or antifungal medicines. Know all the medicines you take. Keep a list of them with you to show your healthcare provider and pharmacist each time you get a new medicine. |
|
How should I take fesoterodine fumarate extended-release tablets?
|
|
What should I avoid while taking fesoterodine fumarate extended-release tablets?
|
|
What are the possible side****effects of fesoterodine fumarate
extended-release tablets? *serious allergic reactions. Symptoms of a serious allergic reaction may include swelling of the face, lips, throat, or tongue. If you have any of these symptoms, you should stop taking fesoterodine fumarate extended-release tablets and get emergency medical help right away. ***inability to empty bladder (urinary retention).**Fesoterodine fumarate extended-release tablets may increase your chances of not being able to empty your bladder if you have bladder outlet obstruction. Tell your healthcare provider right away if you are unable to empty your bladder. ***central nervous system (CNS) effects.**Talk to your healthcare provider right away if you get any of these side effects: headache, dizziness, and drowsiness. *worsening of Myasthenia Gravis symptoms. The most common side effects of fesoterodine fumarate extended-release tablets in adults include:
Talk to your healthcare provider about any side effect that bothers you or that does not go away. These are not all the possible side effects of fesoterodine fumarate extended**-**release tablets. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
How****should I store fesoterodine fumarate extended-release tablets?
|
|
General information about the safe and effective use of fesoterodine fumarate extended-release tablets. Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use fesoterodine fumarate extended**-release tablets for a condition for which it was not prescribed. Do not give fesoterodine fumarate extended-release tablets to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about fesoterodine fumarate extended-**release tablets that is written for health professionals. |
|
What are the ingredients in****fesoterodine fumarate extended-release tablets? Active ingredient: fesoterodine fumarate. Inactive ingredients: FD&C Blue #2/indigo carmine aluminum lake, glyceryl dibehenate, hypromellose, lactose monohydrate, lecithin (soya), microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide, and xylitol. Pediatric use information is approved for Pfizer Inc.’s TOVIAZ® (fesoterodine fumarate) extended-release tablets. However, due to Pfizer Inc.’s marketing exclusivity rights, this drug product is not labeled with that information. All brands listed are the trademarks of their respective owners and are not trademarks of Aurobindo Pharma Limited. Distributed by: Manufactured by: For more information, call Aurobindo Pharma USA, Inc. at 1-866-850-2876. |
This Patient Information has been approved by the U.S. Food and Drug Administration.
Revised: 04/2024
