Registrants (1)
171714327
Manufacturing Establishments (1)
Bryant Ranch Prepack
Bryant Ranch Prepack
171714327
Products (1)
hydroxyzine pamoate
71335-1058
ANDA086183
ANDA (C73584)
ORAL
March 29, 2023
Drug Labeling Information
DESCRIPTION SECTION
DESCRIPTION
Hydroxyzine pamoate is a light yellow, practically odorless powder practically insoluble in water and methanol and freely soluble in dimethylformamide. It is chemically designated as (±)-2-[2-[4-(p-Chloro-α- phenylbenzyl)-1-piperazinyl]ethoxy]ethanol 4,4’-methylenebis[3-hydroxy-2-naphthoate] (1:1) [10246-75-0] and can be structurally represented as follows:
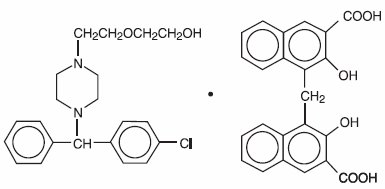
C21H27CIN2O2•C23H16O6
M.W. 763.27
Each capsule, for oral administration, contains hydroxyzine pamoate equivalent to hydroxyzine hydrochloride 25 mg or 50 mg.
In addition, each capsule contains the following inactive ingredients: colloidal silicon dioxide, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, sodium starch glycolate (potato), and sodium lauryl sulfate.
The capsule shell contains the following ingredients: D&C Yellow #10, FD&C Green #3, FD&C Yellow #6, gelatin, and titanium dioxide.
The edible imprinting ink contains the following ingredients: black iron oxide, D&C Yellow #10, FD&C Blue #1, FD&C Blue #2, FD&C Red #40, propylene glycol, and shellac glaze.
