doxycycline hyclate
These highlights do not include all the information needed to use DOXYCYCLINE HYCLATE TABLETS safely and effectively. See full prescribing information for DOXYCYCLINE HYCLATE TABLETS. DOXYCYCLINE HYCLATE tablets, for oral useInitial U.S. Approval: 1967
f234f419-42da-4a6d-82c6-92106cd77c67
HUMAN PRESCRIPTION DRUG LABEL
Sep 19, 2025
Lupin Pharmaceuticals, Inc.
DUNS: 089153071
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
doxyclycline hyclate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
doxyclycline hyclate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
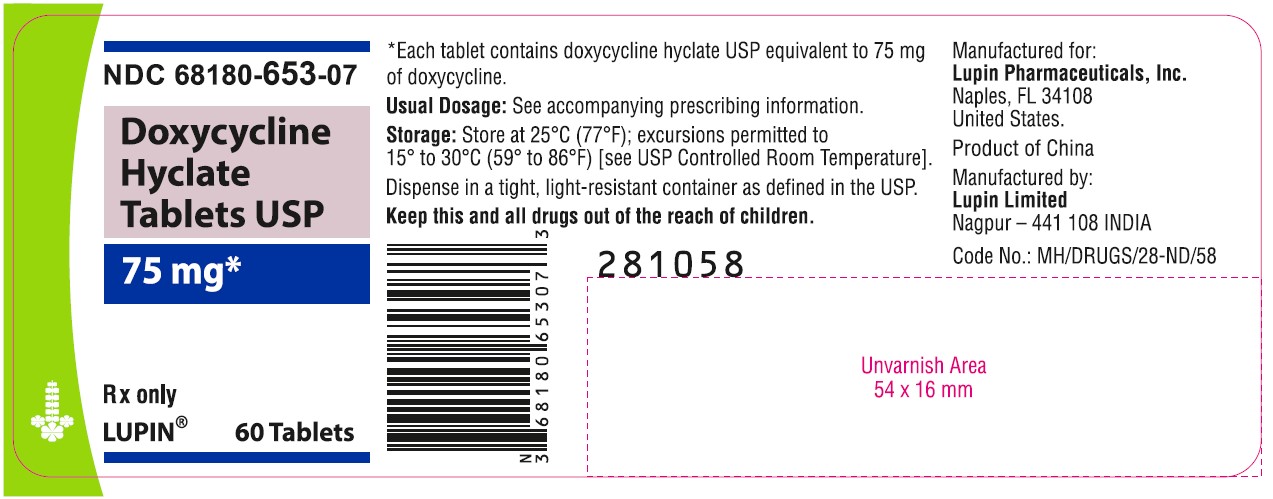
NDC 68180-653-07
Doxycycline Hyclate Tablets USP
75 mg
Rx only
Bottle of 60 Tablets
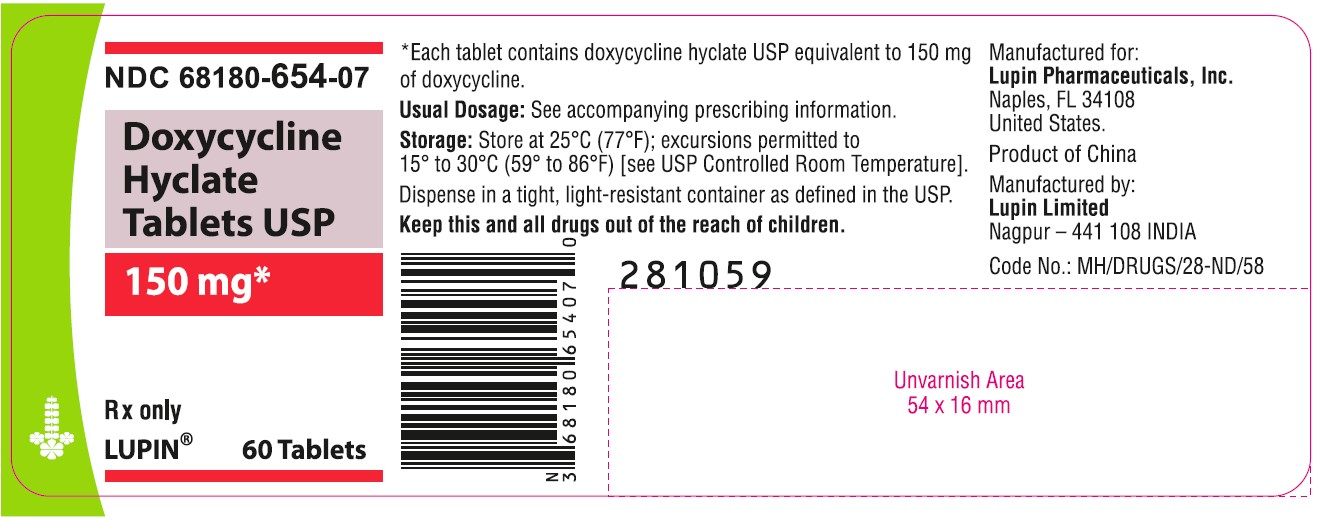
NDC 68180-654-07
Doxycycline Hyclate Tablets USP
150 mg
Rx only
Bottle of 60 Tablets
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
1.1 Rickettsial Infections
Doxycycline hyclate tablets are indicated for treatment of Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsial pox, and tick fevers caused by Rickettsiae.
1.2 Sexually Transmitted Infections
Doxycycline hyclate tablets are indicated for treatment of the following sexually transmitted infections:
- Uncomplicated urethral, endocervical or rectal infections caused by Chlamydia trachomatis .
- Nongonococcal urethritis caused by Ureaplasma urealyticum .
- Lymphogranuloma venereum caused by Chlamydia trachomatis .
- Granuloma inguinale caused by Klebsiella granulomatis .
- Uncomplicated gonorrhea caused by Neisseria gonorrhoeae .
- Chancroid caused by Haemophilus ducreyi .
1.3 Respiratory Tract Infections
Doxycycline hyclate tablets are indicated for treatment of the following respiratory tract infections:
- Respiratory tract infections caused by Mycoplasma pneumoniae .
- Psittacosis (ornithosis) caused by Chlamydophila psittaci .
- Because many strains of the following groups of microorganisms have been shown to be resistant to doxycycline, culture and susceptibility testing are recommended.
- Doxycycline is indicated for treatment of infections caused by the following microorganisms, when bacteriological testing indicates appropriate susceptibility to the drug:
- Respiratory tract infections caused by Haemophilus influenzae .
- Respiratory tract infections caused by Klebsiella species .
- Upper respiratory infections caused by Streptococcus pneumoniae .
1.4 Specific Bacterial Infections
Doxycycline hyclate tablets are indicated for treatment of the following specific bacterial infections:
- Relapsing fever due to Borrelia recurrentis .
- Plague due to Yersinia pestis .
- Tularemia due to Francisella tularensis .
- Cholera caused by Vibrio cholerae .
- Campylobacter fetus infections caused by Campylobacter fetus .
- Brucellosis due to Brucella species (in conjunction with streptomycin).
- Bartonellosis due to Bartonella bacilliformis.
Because many strains of the following groups of microorganisms have been shown to be resistant to doxycycline, culture and susceptibility testing are recommended.
Doxycycline hyclate tablets are indicated for treatment of infections caused by the following gram-negative microorganisms, when bacteriological testing indicates appropriate susceptibility to the drug:
- Escherichia coli
- Enterobacter aerogenes
- Shigella species
- Acinetobacter species
- Urinary tract infections caused by Klebsiella species.
1.5 Ophthalmic Infections
Doxycycline hyclate tablets are indicated for treatment of the following ophthalmic infections:
- Trachoma caused by Chlamydia trachomatis , although the infectious agent is not always eliminated as judged by immunofluorescence.
- Inclusion conjunctivitis caused by Chlamydia trachomatis .
1.6 Anthrax Including Inhalational Anthrax (Post-Exposure)
Doxycycline hyclate tablets are indicated for the treatment of Anthrax due to Bacillus anthracis, including inhalational anthrax (post-exposure); to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis.
1.7 Alternative Treatment for Selected Infections when Penicillin is
Contraindicated
Doxycycline hyclate tablets are indicated as an alternative treatment for the following selected infections when penicillin is contraindicated:
- Syphilis caused by Treponema pallidum .
- Yaws caused by Treponema pallidum subspecies pertenue .
- Listeriosis due to Listeria monocytogenes .
- Vincent's infection caused by Fusobacterium fusiforme.
- Actinomycosis caused by Actinomyces israelii .
- Infections caused by Clostridium species.
1.8 Adjunctive Therapy for Acute Intestinal Amebiasis and Severe Acne
In acute intestinal amebiasis, doxycycline hyclate tablets may be a useful adjunct to amebicides. In severe acne, doxycycline hyclate tablets may be useful adjunctive therapy.
1.9 Prophylaxis of Malaria
Doxycycline hyclate tablets are indicated for the prophylaxis of malaria due to Plasmodium falciparum in short-term travelers (less than 4 months) to areas with chloroquine and/or pyrimethamine-sulfadoxine resistant strains [see Dosage and Administration (2.4) and Patient Counseling Information (17)].
1.10 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of doxycycline hyclate tablets and other antibacterial drugs, doxycycline hyclate tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Doxycycline hyclate tablet is a tetracycline class drugs indicated for:
- Rickettsial infections (1.1)
- Sexually transmitted infections (1.2)
- Respiratory tract infections (1.3)
- Specific bacterial infections (1.4)
- Ophthalmic infections (1.5)
- Anthrax, including inhalational anthrax (post-exposure) (1.6)
- Alternative treatment for selected infections when penicillin is contraindicated (1.7)
- Adjunctive therapy for acute intestinal amebiasis and severe acne (1.8)
- Prophylaxis of malaria (1.9)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of doxycycline hyclate tablets and other antibacterial drugs, doxycycline hyclate tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.10)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Tooth Development
The use of doxycycline hyclate during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term use of the drugs of the tetracycline class, but it has been observed following repeated short-term courses. Enamel hypoplasia has also been reported with drugs of the tetracycline class. Advise the patient of the potential risk to the fetus if doxycycline hyclate is used during pregnancy [see Use in Specific Populations (8.1, 8.4)]. Use doxycycline hyclate in pediatric patients 8 years of age or less only when the potential benefits are expected to outweigh the risks in severe or life-threatening conditions (e.g., anthrax, Rocky Mountain spotted fever), particularly when there are no alternative therapies.
5.2 Inhibition of Bone Growth
The use of doxycycline hyclate during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth. All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued. Advise the patient of the potential risk to the fetus if doxycycline hyclate is used during pregnancy [see Use in Specific Populations (8.1, 8.4)].
5.3 Clostridioides difficile Associated Diarrhea
Clostridioides difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including doxycycline hyclate, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.4 Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema.
5.5 Severe Skin Reactions
Severe skin reactions, such as exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported in patients receiving doxycycline Fixed drug eruptions have occurred with doxycycline and have been associated with worsening severity upon subsequent administrations, including generalized bullous fixed drug eruption [See Adverse Reactions (6)].If severe skin reactions occur, discontinue doxycycline hyclate immediately and institute appropriate therapy.
5.6 Intracranial Hypertension
Intracranial hypertension (IH, pseudotumor cerebri) has been associated with the use of tetracyclines including doxycycline hyclate. Clinical manifestations of IH include headache, blurred vision, diplopia, and vision loss; papilledema can be found on fundoscopy. Women of childbearing age who are overweight or have a history of IH are at greater risk for developing tetracycline associated IH. Concomitant use of isotretinoin and doxycycline hyclate should be avoided because isotretinoin is also known to cause pseudotumor cerebri.
Although IH typically resolves after discontinuation of treatment, the possibility for permanent visual loss exists. If visual disturbance occurs during treatment, prompt ophthalmologic evaluation is warranted. Since intracranial pressure can remain elevated for weeks after drug cessation patients should be monitored until they stabilize.
5.7 Antianabolic Action
The antianabolic action of the tetracyclines may cause an increase in BUN. Studies to date indicate that this does not occur with the use of doxycycline in patients with impaired renal function.
5.8 Incomplete Suppression of Malaria
Doxycycline offers substantial but not complete suppression of the asexual blood stages of Plasmodium strains.
Doxycycline does not suppress P. falciparum's sexual blood stage gametocytes. Subjects completing this prophylactic regimen may still transmit the infection to mosquitoes outside endemic areas.
5.9 Development of Drug-Resistant Bacteria
Prescribing doxycycline hyclate in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
5.10 Potential for Microbial Overgrowth
Doxycycline hyclate may result in overgrowth of non-susceptible organisms, including fungi. If such infections occur, discontinue use and institute appropriate therapy.
5.11 Laboratory Monitoring for Long-Term Therapy
In long-term therapy, periodic laboratory evaluation of organ systems, including hematopoietic, renal and hepatic studies should be performed.
- The use of doxycycline hyclate during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown) and enamel hypoplasia Advise the patient of the potential risk to the fetus during pregnancy. (2.2, 5.1, 8.1,8.4)
- The use of doxycycline hyclate during the second and third-trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth. Advise the patient of the potential risk to the fetus during pregnancy. (5.2,8.1, 8.4)
- Clostridioides difficile -associated diarrhea (CDAD) has been reported. Evaluate patients if diarrhea occurs. (5.3)
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Limit sun exposure. (5.4)
- Overgrowth of non-susceptible organisms, including fungi, may occur. If such infections occur, discontinue use and institute appropriate therapy. (5.10)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions have been identified during clinical trials or post-approval use of tetracycline-class drugs, including doxycycline. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal
Anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, inflammatory lesions (with monilial overgrowth) in the anogenital region, and pancreatitis. Hepatotoxicity has been reported. These reactions have been caused by both the oral and parenteral administration of tetracyclines. Superficial discoloration of the adult permanent dentition, reversible upon drug discontinuation and professional dental cleaning has been reported. Permanent tooth discoloration and enamel hypoplasia may occur with drugs of the tetracycline class when used during tooth development [See Warnings and Precautions (5.1)]. Instances of esophagitis and esophageal ulcerations have been reported in patients receiving capsule and tablet forms of drugs in the tetracycline-class. Most of these patients took medications immediately before going to bed [see Dosage and Administration (2.1)].
Skin
Maculopapular and erythematous rashes, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis, and erythema multiforme, and fixed drug eruption have been reported. Photosensitivity has been reported [see Warnings and Precautions (5.3)]
Renal
Rise in BUN has been reported and is apparently dose-related [see Warnings and Precautions (5.7)].
Hypersensitivity reactions
Urticaria, angioneurotic edema, anaphylaxis, anaphylactoid purpura, serum sickness, pericarditis, exacerbation of systematic lupus erythematosus and drug reaction with eosinophilia and systematic symptoms (DRESS).
Blood
Hemolytic anemia, thrombocytopenia, neutropenia, and eosinophilia have been reported.
Intracranial Hypertension
Intracranial hypertension (IH, pseudotumor cerebri) has been associated with the use of tetracyclines [see Warnings and Precautions (5.6)].
Thyroid Gland Changes
When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of thyroid glands. No abnormalities of thyroid function are known to occur.
Psychiatric: Depression, anxiety, suicidal ideation, insomnia, abnormal dreams, hallucination.
Adverse reactions observed in patients receiving tetracyclines include anorexia, nausea, vomiting, diarrhea, rash, photosensitivity, urticaria, and hemolytic anemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Lupin Pharmaceuticals, Inc. at 1-800-399-2561, or FDA at 1-800-FDA-1088 or****www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Anticoagulant Drugs
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
7.2 Penicillin
Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracyclines, including doxycycline hyclate in conjunction with penicillin.
7.3 Antacids and Iron Preparations
Absorption of tetracyclines, including doxycycline hyclate is impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate, and iron-containing preparations.
7.4 Oral Contraceptives
Concurrent use of tetracyclines, including doxycycline hyclate may render oral contraceptives less effective.
7.5 Barbiturates and Anti-Epileptics
Barbiturates, carbamazepine, and phenytoin decrease the half-life of doxycycline.
7.7 Drug and Laboratory Test Interactions
False elevations of urinary catecholamines may occur due to interference with the fluorescence test.
- Patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage. (7.1)
- Avoid co-administration of tetracyclines with penicillin. (7.2)
- Absorption of tetracyclines, including doxycycline hyclate is impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate and iron-containing preparations. (7.3)
- Concurrent use of tetracyclines, including doxycycline hyclate may render oral contraceptives less effective. (7.4)
- Barbiturates, carbamazepine and phenytoin decrease the half-life of doxycycline. (7.5)
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Important Administration and Safety Information for Patients and Caregivers
Advise patients taking doxycycline hyclate for malaria prophylaxis:
- that no present-day antimalarial agent, including doxycycline, guarantees protection against malaria.
- to avoid being bitten by mosquitoes by using personal protective measures that help avoid contact with mosquitoes, especially from dusk to dawn (for example, staying in well-screened areas, using mosquito nets, covering the body with clothing, and using an effective insect repellent).
- that doxycycline prophylaxis:
- should begin 1 day to 2 days before travel to the malarious area,
- should be continued daily while in the malarious area and after leaving the malarious area,
- should be continued for 4 further weeks to avoid development of malaria after returning from an endemic area,
- should not exceed 4 months.
Advise all patients taking doxycycline hyclate:
- that doxycycline hyclate tablets (150 mg) can be broken into two-thirds or one-third at the scored lines to provide 100 mg or 50 mg strength doses, respectively.
- to avoid excessive sunlight or artificial ultraviolet light while receiving doxycycline and to discontinue therapy if phototoxicity (for example, skin eruptions, etc.) occurs. Sunscreen or sunblock should be considered [see Warnings and Precautions (5.4)] .
- to drink fluids liberally along with doxycycline hyclate to reduce the risk of esophageal irritation and ulceration [see Adverse Reactions (6)].
- that the absorption of tetracyclines is reduced when taken with foods, especially those that contain calcium [see Drug Interactions (7.3)]. However, the absorption of doxycycline is not markedly influenced by simultaneous ingestion of food or milk [see Clinical Pharmacology (12.3)].
- that if gastric irritation occurs, doxycycline hyclate may be given with food or milk [see Clinical Pharmacology (12.3)].
- that the absorption of tetracyclines is reduced when taken with antacids containing aluminum, calcium or magnesium, bismuth subsalicylate, and iron-containing preparations [see Drug Interactions (7.3)].
- that the use of doxycycline might increase the incidence of vaginal candidiasis.
Tooth Discoloration and Inhibition of Bone Growth
Advise patients that doxycycline hyclate, like other tetracycline-class drugs, may cause permanent tooth discoloration of deciduous teeth and reversible inhibition of bone growth when administered during pregnancy. Tell your healthcare provider right away if you become pregnant during treatment [see Warnings and Precautions (5.1, 5.2) and Use in Specific Populations (8.1, 8.4)].
Lactation
Advise women not to breastfeed during treatment with doxycycline hyclate and for 5 days after the last dose [see Use in Specific Populations (8.2)].
Diarrhea
Advise patients that diarrhea is a common problem caused by antibacterial drugs which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of antibacterial. If this occurs, patients should contact their physician as soon as possible.
Development of Resistance
Counsel patients that antibacterial drugs including doxycycline hyclate should only be used to treat bacterial infections. They do not treat viral infections (for example, the common cold). When doxycycline hyclate are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by doxycycline hyclate or other antibacterial drugs in the future.
The brands listed are trademarks of their respective owners and are not trademarks of Lupin Pharmaceuticals, Inc. The makers of these brands are not affiliated with and do not endorse Lupin Pharmaceuticals, Inc. or its products.

Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
Manufactured by:
Lupin Limited
Nagpur 441 108
INDIA
Revised: April 2025
DESCRIPTION SECTION
11 DESCRIPTION
Doxycycline Hyclate Tablets USP contain doxycycline hyclate, a tetracycline class drug synthetically derived from oxytetracycline, in an immediate release formulation for oral administration.
The molecular formula of doxycycline hyclate is (C22H24N2O8●HCl)2●C2H6O●H2O and the molecular weight of doxycycline hyclate is 1025.87. The chemical name for doxycycline hyclate is: 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride, compound with ethyl alcohol (2:1), monohydrate.
The structural formula for doxycycline hyclate is:
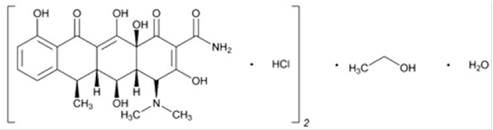
Figure 1: Structure of Doxycycline Hyclate
Doxycycline hyclate is a yellow crystalline powder soluble in water and in solutions of alkali hydroxides and carbonates.
Doxycycline Hyclate Tablets USP:
Doxycycline hyclate tablets USP are available as 75 mg and 150 mg tablets. Each 75 mg tablet contains 86.6 mg of doxycycline hyclate equivalent to 75 mg of doxycycline. Each 150 mg tablet contains 173.2 mg of doxycycline hyclate equivalent to 150 mg of doxycycline.
Inactive ingredients in the tablet formulation are: croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose and sodium lauryl sulfate. Film-coating contains: FD & C Blue # 1 / Brilliant Blue FCF Aluminum Lake (75 mg Tablet), FD & C Yellow # 6 /Sunset Yellow FCF Aluminum Lake (75 mg Tablet), FD & C Blue #2 / Indigo Carmine AL (150 mg Tablet), iron oxide yellow (150 mg Tablet), polyethylene glycol, polyvinyl alcohol, talc, titanium dioxide. Doxycycline hyclate tablets USP, 75 mg contain 0.34 mg (0.0146 mEq) of sodium. Doxycycline hyclate tablets USP, 150 mg contain 0.68 mg (0.0295 mEq) of sodium.
Doxycycline hyclate tablets USP meets USP Dissolution Test 3.
SPL PATIENT PACKAGE INSERT SECTION
FDA-Approved Patient Labeling
Instructions for Use
Doxycycline Hyclate (DOX-i-SYE-kleen HYE-klate)
Tablets
for oral use
Read this Instructions for Use before you start using doxycycline hyclate tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Note:
- Your healthcare provider may need to change your dose of doxycycline hyclate tablets during treatment as needed.
- Doxycycline hyclate tablets can be taken whole or broken at scored lines.
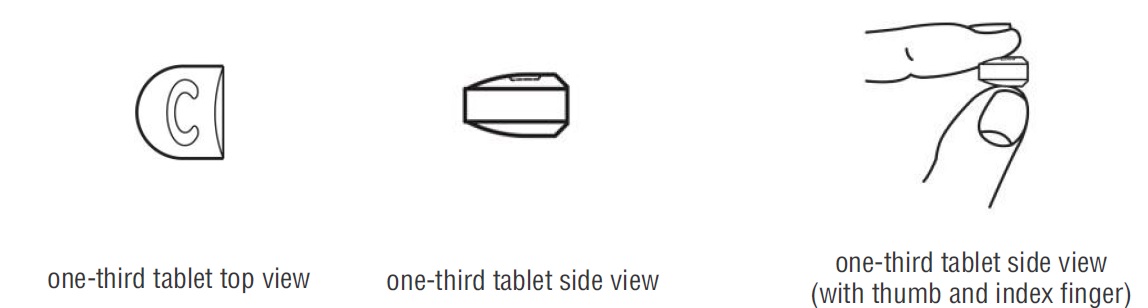
- Doxycycline hyclate tablets are marked with scored lines and may be broken at these scored lines to provide the following doses:
150 mg treatment (take the entire whole tablet)
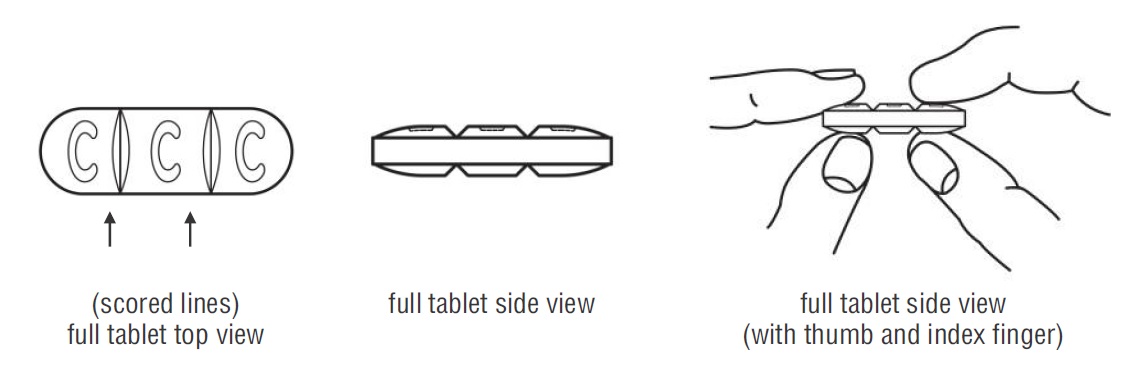
100 mg treatment (take two-thirds of the tablet)
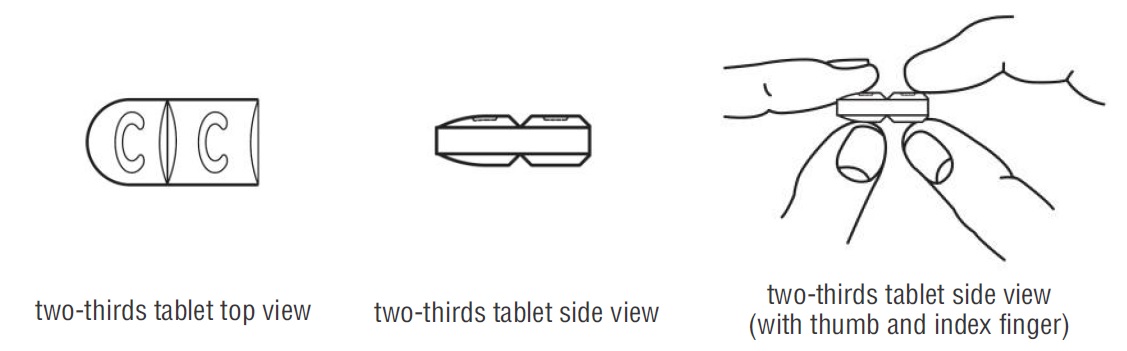
50 mg treatment (take one-third of the tablet)
How to break your doxycycline hyclate tablet:
- Hold the tablet between your thumb and index finger close to the scored line for your dose of doxycycline hyclate tablet as shown above.
- Apply enough pressure to break the tablet at the scored line. *Do not break the doxycycline hyclate tablet in any other way.
Rx only
This Instructions for Use has been approved by the U.S. Food and Drug Administration.

Manufactured for:
Lupin Pharmaceuticals, Inc.
Naples, FL 34108
United States
Manufactured by:
Lupin Limited
Nagpur 441 108
INDIA
Revised: April 2025 ID: 280400
RECENT MAJOR CHANGES SECTION
RECENT MAJOR CHANGES
Warnings and Precautions, Severe Skin Reactions (5.5) 3/2025
Warnings and Precautions, Severe Skin Reactions (5.5) 3/2025
