Baytril
a1ada9f6-24e2-4287-8b83-00e2b68666e5
PRESCRIPTION ANIMAL DRUG LABEL
Aug 25, 2025
Elanco US Inc.
DUNS: 966985624
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Enrofloxacin
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (2)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel - Bottle Label
Elanco
Baytril**®**
(enrofloxacin)
Antibacterial Injectable Solution 2.27%
For The Treatment Of Susceptible Bacterial
Pathogens In Dogs Only
20 mL
CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the
order
of a licensed veterinarian.
Federal law prohibits the extralabel use of this drug in food-producing animals.
Manufactured for: Elanco US Inc.
Greenfield, IN 46140 U.S.A.
Approved by FDA under NADA # 140-913, Made in Germany

Elanco
Baytril**®**
(enrofloxacin)
Antibacterial Injectable Solution 2.27%
For The Treatment Of Susceptible Bacterial
Pathogens In Dogs Only
20 mL
CAUTION: Federal (U.S.A.) law restricts this drug to use by or on the
order
of a licensed veterinarian.
Federal law prohibits the extralabel use of this drug in food-producing animals.
Manufactured for: Elanco US Inc.
Greenfield, IN 46140 U.S.A.
Approved by FDA under NADA # 140-913, Product of China

INDICATIONS & USAGE SECTION
INDICATIONS:
Baytril® (brand of enrofloxacin) Injectable Solution is indicated for the management of diseases in dogs associated with bacteria susceptible to enrofloxacin.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS:
Enrofloxacin is contraindicated in dogs known to be hypersensitive to quinolones.
Based on the studies discussed under the section on Animal Safety Summary, the use of enrofloxacin is contraindicated in small and medium breeds of dogs during the rapid growth phase (between 2 and 8 months of age). The safe use of enrofloxacin has not been established in large and giant breeds during the rapid growth phase. Large breeds may be in this phase for up to one year of age and the giant breeds for up to 18 months. In clinical field trials utilizing a daily oral dose of 5.0 mg/kg, there were no reports of lameness or joint problems in any breed. However, controlled studies with histological examination of the articular cartilage have not been conducted in the large or giant breeds.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS:
No drug-related side effects were reported in 122 clinical cases treated with Baytril (enrofloxacin) Injectable Solution followed by Baytril Tablets at 5.0 mg/kg per day.
For medical emergencies or to report adverse reactions, call 888-545-5973. For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae.
DRUG INTERACTIONS SECTION
DRUG INTERACTIONS:
Concomitant therapy with other drugs that are metabolized in the liver may reduce the clearance rates of the quinolone and the other drug.
Enrofloxacin has been administered to dogs at a daily dosage rate of 10 mg/kg concurrently with a wide variety of other health products including anthelmintics (praziquantel, febantel), insecticides (pyrethrins), heartworm preventatives (diethylcarbamazine) and other antibiotics (ampicillin, gentamicin sulfate, penicillin). No incompatibilities with other drugs are known at this time.
SPL UNCLASSIFIED SECTION
ANIMAL SAFETY SUMMARY:
Adult dogs receiving enrofloxacin orally at a daily dosage rate 52 mg/kg for 13 weeks had only isolated incidences of vomition and inappetence. Adult dogs receiving the tablet formulation for 30 consecutive days at a daily treatment of 25 mg/kg did not exhibit significant clinical signs nor were there effects upon the clinical chemistry, hematological or histological parameters. Daily doses of 125 mg/kg for up to 11 days induced vomition, inappetence, depression, difficult locomotion and death while adult dogs receiving 50 mg/kg/day for 14 days had clinical signs of vomition and inappetence.
Adult dogs dosed intramuscularly for three treatments at 12.5 mg/kg followed by 57 oral treatments at 12.5 mg/kg, all at 12 hour intervals, did not exhibit either significant clinical signs or effects upon the clinical chemistry, hematological or histological parameters.
Oral treatment of 15 to 28 week old growing puppies with daily dosage rates of 25 mg/kg has induced abnormal carriage of the carpal joint and weakness in the hindquarters. Significant improvement of clinical signs is observed following drug withdrawal. Microscopic studies have identified lesions of the articular cartilage following 30 day treatments at either 5,15 or 25 mg/kg in this age group. Clinical signs of difficult ambulation or associated cartilage lesions have not been observed in 29 to 34 week old puppies following daily treatments of 25 mg/kg for 30 consecutive days nor in 2 week old puppies with the same treatment schedule.
Tests indicated no effect on circulating microfilariae or adult heart-worms (Dirofilaria immitis) when dogs were treated at a daily dosage rate of 15 mg/kg for 30 days. No effect on cholinesterase values was observed.
No adverse effects were observed on reproductive parameters when male dogs received 10 consecutive daily treatments of 15 mg/kg/day at 3 intervals (90, 45 and 14 days) prior to breeding or when female dogs received 10 consecutive daily treatments of 15 mg/kg/day at 4 intervals: between 30 and 0 days prior to breeding, early pregnancy (between 10th & 30th days), late pregnancy (between 40th & 60th days), and during lactation (the first 28 days).
DESCRIPTION SECTION
DESCRIPTION:
Enrofloxacin is a synthetic chemotherapeutic agent from the class of the quinolone carboxylic acid derivatives. It has antibacterial activity against a broad spectrum of Gram negative and Gram positive bacteria (See Tables I and II). Each mL of injectable solution contains: enrofloxacin 22.7 mg, n-butyl alcohol 30 mg, potassium hydroxide for pH adjustment and water for injection, q.s.
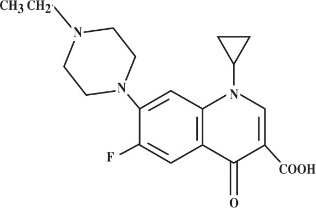
CHEMICAL NOMENCLATURE AND STRUCTURAL FORMULA:
1-cyclopropyl-7-(4-ethyl-1-piperazinyl)-6-fluoro-1,4-dihydro-4-oxo-3-quinolinecarboxylic acid.
WARNINGS SECTION
WARNINGS:
For use in animals only. The use of this product in cats may result in Retinal Toxicity. Keep out of reach of children.
Avoid contact with eyes. In case of contact, immediately flush eyes with copious amounts of water for 15 minutes. In case of dermal contact, wash skin with soap and water. Consult a physician if irritation persists following ocular or dermal exposure. Individuals with a history of hypersensitivity to quinolones should avoid this product. In humans, there is a risk of user photosensitization within a few hours after excessive exposure to quinolones. If excessive accidental exposure occurs, avoid direct sunlight.
For customer service or to obtain product information, including Safety Data Sheet, call 888-545-5973.
PRECAUTIONS SECTION
PRECAUTION:
Quinolone-class drugs should be used with caution in animals with known or suspected Central Nervous System (CNS) disorders. In such animals, quinolones have, in rare instances, been associated with CNS stimulation which may lead to convulsive seizures.
Quinolone-class drugs have been associated with cartilage erosions in weight- bearing joints and other forms of arthropathy in immature animals of various species.
The use of fluoroquinolones in cats has been reported to adversely affect the retina. Such products should be used with caution in cats.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION:
Baytril Injectable Solution may be used as the initial dose at 2.5 mg/kg. It should be administered intramuscularly (IM) as a single dose, followed by initiation of Baytril Tablet therapy.
Baytril Injectable Solution may be administered as follows:
|
*The initial Baytril Injectable administration should be followed 12 hours later by initiation of Baytril Tablet therapy. | |
|
Weight |
Baytril |
|
9.1 kg |
1.00 mL |
|
27.2 kg |
3.00 mL |
The lower limit of the dose range was based on efficacy studies in dogs where enrofloxacin was administered at 2.5 mg/kg twice daily. Target animal safety and toxicology studies were used to establish the upper limit of the dose range and treatment duration.
STORAGE AND HANDLING SECTION
STORAGE:
Protect from direct sunlight. Do not freeze. Store at or below 25°C (77°F), excursions permitted up to 40°C (104°F).
Use within 90 days of first puncture and puncture a maximum of 20 times. Any product remaining after 20 punctures or more than 90 days after initial puncture should be discarded.
HOW SUPPLIED SECTION
HOW SUPPLIED:
Baytril Injectable Solution
22.7 mg/mL Vial Size
20 mL
REFERENCES SECTION
REFERENCES:
Dougherty, T.J. and Saukkonen, J.J. Membrane Permeability Changes Associated with DNA Gyrase Inhibitors in Escherichia coli. Antimicrob.Agents and Chemoth., V. 28, Aug. 1985:200-206.
Walker, R.D., et al. Pharmacokinetic Evaluation of Enrofloxacin Administered Orally to Healthy Dogs. Am.J. Res., V. 53, No. 12, Dec. 1992: 2315-2319.
90206975_PA600218X Revised: January 2022
Approved by FDA under NADA # 140-913
Baytril is sold by Elanco or its affiliates and is not a product of Bayer. The product name Baytril is owned by Bayer and used under license. Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2022 Elanco or its affiliates.
Elanco™
Manufactured for:
Elanco US Inc.
Greenfield, IN 46140 U.S.A.
