Valsartan
These highlights do not include all the information needed to use VALSARTAN TABLETS safely and effectively. See full prescribing information for VALSARTAN TABLETS. VALSARTAN tablets, for oral useInitial U.S. Approval:1996
5ee97546-2a7c-40fa-9e05-b7993a72dfbf
HUMAN PRESCRIPTION DRUG LABEL
Jul 2, 2023
SQUARE PHARMACEUTICALS LIMITED
DUNS: 731487153
Products 4
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Valsartan
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Valsartan
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
Valsartan
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Valsartan
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
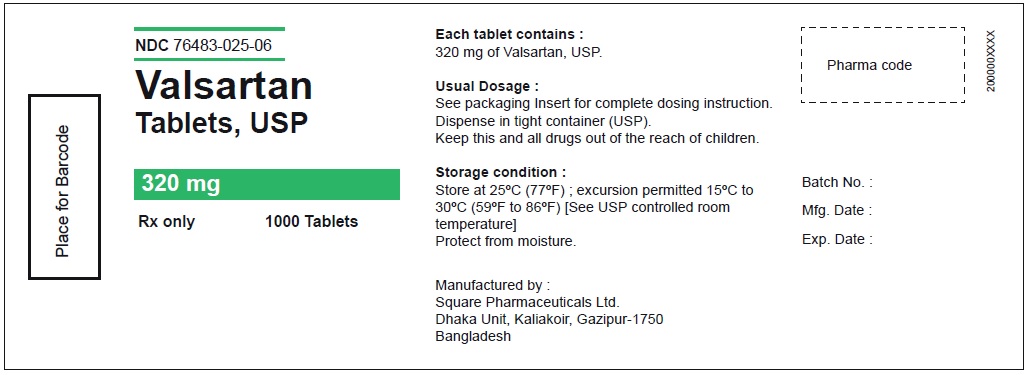
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package Label – 320 mg
Rx Only
NDC 76483-025-06
Valsartan Tablets, USP
320 mg
1000 Tablets
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Valsartan can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue valsartan as soon as possible. [see Use in Specific Populations (8.1)].
5.2 Hypotension
Excessive hypotension was rarely seen (0.1%) in patients with uncomplicated hypertension treated with Valsartan alone. In patients with an activated renin-angiotensin system, such as volume- and/or salt-depleted patients receiving high doses of diuretics, symptomatic hypotension may occur. This condition should be corrected prior to administration of Valsartan, or the treatment should start under close medical supervision.
Patients with heart failure or post-myocardial infarction patients given Valsartan commonly have some reduction in blood pressure, but discontinuation of therapy because of continuing symptomatic hypotension usually is not necessary when dosing instructions are followed. In controlled trials in heart failure patients, the incidence of hypotension in valsartan-treated patients was 5.5% compared to 1.8% in placebo-treated patients. In the VALsartan In Acute myocardial iNfarcTion trial (VALIANT), hypotension in post-myocardial infarction patients led to permanent discontinuation of therapy in 1.4% of valsartan-treated patients and 0.8% of captopril-treated patients.
If excessive hypotension occurs, place the patient in the supine position and, if necessary, give intravenous normal saline. A transient hypotensive response is not a contraindication to further treatment, which usually can be continued without difficulty once the blood pressure has stabilized.
5.3 Impaired Renal Function
Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system and by diuretics. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g. patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, or volume depletion) may be at particular risk of developing acute renal failure on Valsartan. Monitor renal function periodically in these patients. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on Valsartan. [See Drug Interactions (7)].
5.4 Hyperkalemia
Some patients with heart failure have developed increases in potassium. These effects are usually minor and transient, and they are more likely to occur in patients with pre-existing renal impairment. Dosage reduction and/or discontinuation of Valsartan may be required. [see Adverse Reactions (6.1)]
- Observe for signs and symptoms of hypotension (5.2)
- Monitor renal function and potassium in susceptible patients (5.3, 5.4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reactions rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adult Hypertension
Valsartan has been evaluated for safety in more than 4,000 patients, including over 400 treated for over 6 months, and more than 160 for over 1 year. Adverse reactions have generally been mild and transient in nature and have only infrequently required discontinuation of therapy. The overall incidence of adverse reactions with Valsartan was similar to placebo.
The overall frequency of adverse reactions was neither dose-related nor related to gender, age, race, or regimen. Discontinuation of therapy due to side effects was required in 2.3% of valsartan patients and 2.0% of placebo patients. The most common reasons for discontinuation of therapy with Valsartan were headache and dizziness.
The adverse reactions that occurred in placebo-controlled clinical trials in at least 1% of patients treated with Valsartan and at a higher incidence in valsartan (n=2,316) than placebo (n=888) patients included viral infection (3% vs. 2%), fatigue (2% vs. 1%), and abdominal pain (2% vs. 1%).
In trials in which valsartan was compared to an ACE inhibitor with or without placebo, the incidence of dry cough was significantly greater in the ACE- inhibitor group (7.9%) than in the groups who received valsartan (2.6%) or placebo (1.5%). In a 129-patient trial limited to patients who had dry cough when they had previously received ACE inhibitors, the incidences of cough in patients who received valsartan, HCTZ, or lisinopril were 20%, 19%, and 69% respectively (p <0.001).
Dose-related orthostatic effects were seen in less than 1% of patients. An increase in the incidence of dizziness was observed in patients treated with Valsartan 320 mg (8%) compared to 10 to 160 mg (2% to 4%).
Pediatric Hypertension
Valsartan has been evaluated for safety in over 400 patients aged 6 to 17 years. No relevant differences were identified between the adverse experience profile for pediatric patients and that previously reported for adult patients. Hyperkalemia was more frequently observed in pediatric patients with underlying chronic kidney disease (CKD).
Heart Failure
In the Valsartan Heart Failure Trial (Val-HeFT), comparing valsartan in total daily doses up to 320 mg (n=2,506) to placebo (n=2,494), 10% of valsartan patients discontinued for adverse reactions vs. 7% of placebo patients.
The table shows adverse reactions in double-blind short-term heart failure trials, including the first 4 months of the Valsartan Heart Failure Trial, with an incidence of at least 2% that were more frequent in valsartan-treated patients than in placebo-treated patients. All patients received standard drug therapy for heart failure, frequently as multiple medications, which could include diuretics, digitalis, beta-blockers. About 93% of patients received concomitant ACE inhibitors.
|
** Valsartan (n=3,282)** |
** Placebo (n=2,740)** | |
|
Dizziness |
17% |
9% |
|
Hypotension |
7% |
2% |
|
Diarrhea |
5% |
4% |
|
Arthralgia |
3% |
2% |
|
Fatigue |
3% |
2% |
|
Back Pain |
3% |
2% |
|
Dizziness, postural |
2% |
1% |
|
Hyperkalemia |
2% |
1% |
|
Hypotension, postural |
2% |
1% |
Discontinuations occurred in 0.5% of valsartan-treated patients and 0.1% of placebo patients for each of the following: elevations in creatinine and elevations in potassium.
Other adverse reactions with an incidence greater than 1% and greater than placebo included headache, nausea, renal impairment, syncope, blurred vision, upper abdominal pain and vertigo.
From the long-term data in the Valsartan Heart Failure Trial, there did not appear to be any significant adverse reactions not previously identified.
Post-Myocardial Infarction
The table shows the percent of patients discontinued in the valsartan and captopril-treated groups in the VALsartan In Acute myocardial iNfarcTion trial (VALIANT) with a rate of at least 0.5% in either of the treatment groups.
Discontinuations due to renal dysfunction occurred in 1.1% of valsartan- treated patients and 0.8% of captopril-treated patients.
|
** Valsartan (n=4,885)** |
** Captopril (n=4,879)** | |
|
Discontinuation for adverse reaction |
5.8% |
7.7% |
|
Adverse reactions | ||
|
Hypotension NOS |
1.4% |
0.8% |
|
Cough |
0.6% |
2.5% |
|
Blood creatinine increased |
0.6% |
0.4% |
|
Rash NOS |
0.2% |
0.6% |
Clinical Laboratory Test Findings
**Creatinine:**In heart failure trials, greater than 50% increases in creatinine were observed in 3.9% of Valsartan-treated patients compared to 0.9% of placebo-treated patients. In post-myocardial infarction patients, doubling of serum creatinine was observed in 4.2% of valsartan-treated patients and 3.4% of captopril-treated patients.
**Neutropenia:**Neutropenia was observed in 1.9% of patients treated with Valsartan and 0.8% of patients treated with placebo.
**Blood Urea Nitrogen (BUN):**In heart failure trials, greater than 50% increases in BUN were observed in 16.6% of Valsartan-treated patients compared to 6.3% of placebo-treated patients. [see Warnings and Precautions (5.3)]
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's Diovan (valsartan) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information.
6.2 Postmarketing Experience
The following additional adverse reactions have been reported in postmarketing use of Valsartan. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: Angioedema has been reported. Some of these patients previously experienced angioedema with other drugs, including ACE inhibitors. Valsartan should not be re-administered to patients who have had angioedema.
Digestive: Elevated liver enzymes and very rare reports of hepatitis
Musculoskeletal: Rhabdomyolysis
Renal: Impaired renal function, renal failure
Dermatologic: Alopecia, bullous dermatitis
Blood and Lymphatic: Thrombocytopenia
Vascular: Vasculitis
Hypertension: Most common adverse reactions are headache, dizziness, viral infection, fatigue and abdominal pain (6.1)
Heart Failure: Most common adverse reactions are dizziness, hypotension, diarrhea, arthralgia, back pain, fatigue and hyperkalemia (6.1)
Post-Myocardial Infarction: Most common adverse reactions which caused patients to discontinue therapy are hypotension, cough and increased blood creatinine (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of carcinogenicity when valsartan was administered in the diet to mice and rats for up to 2 years at doses up to 160 and 200 mg/kg/day, respectively. These doses in mice and rats are about 2.6 and 6 times, respectively, the MRHD on a mg/m2 basis. (Calculations assume an oral dose of 320 mg/day and a 60-kg patient.)
Mutagenicity assays did not reveal any valsartan-related effects at either the gene or chromosome level. These assays included bacterial mutagenicity tests with Salmonella (Ames) and E coli; a gene mutation test with Chinese hamster V79 cells; a cytogenetic test with Chinese hamster ovary cells; and a rat micronucleus test.
Valsartan had no adverse effects on the reproductive performance of male or female rats at oral doses up to 200 mg/kg/day. This dose is 6 times the MRHD on a mg/m2 basis. (Calculations assume an oral dose of 320 mg/day and a 60-kg patient.)
13.2 Animal Toxicology and/or Pharmacology
Daily oral dosing of neonatal/juvenile rats with valsartan at doses as low as 1 mg/kg/day (about 10% of the maximum recommended pediatric dose on a mg/m2basis) from postnatal day 7 to postnatal day 70 produced persistent, irreversible kidney damage. These kidney effects in neonatal rats represent expected exaggerated pharmacological effects that are observed if rats are treated during the first 13 days of life. This period coincides with 36 weeks of gestation in humans, which could occasionally extend up to 44 weeks after conception in humans. In humans, nephrogenesis is thought to be complete around birth; however, maturation of other aspects of kidney function (such as glomerular filtration and tubular function) may continue until approximately 2 years of age. It is unknown whether post-natal use of valsartan before maturation of renal function is complete has long-term deleterious effects on the kidney [see Use in Specific Populations (8.4)].
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Hypertension
Adult Hypertension
The antihypertensive effects of Valsartan were demonstrated principally in 7 placebo-controlled, 4- to 12-week trials (1 in patients over 65 years) of dosages from 10 to 320 mg/day in patients with baseline diastolic blood pressures of 95-115 mmHg. The studies allowed comparison of once-daily and twice-daily regimens of 160 mg/day; comparison of peak and trough effects; comparison (in pooled data) of response by gender, age, and race; and evaluation of incremental effects of hydrochlorothiazide.
Administration of valsartan to patients with essential hypertension results in a significant reduction of sitting, supine, and standing systolic and diastolic blood pressure, usually with little or no orthostatic change.
In most patients, after administration of a single oral dose, onset of antihypertensive activity occurs at approximately 2 hours, and maximum reduction of blood pressure is achieved within 6 hours. The antihypertensive effect persists for 24 hours after dosing, but there is a decrease from peak effect at lower doses (40 mg) presumably reflecting loss of inhibition of angiotensin II. At higher doses, however (160 mg), there is little difference in peak and trough effect. During repeated dosing, the reduction in blood pressure with any dose is substantially present within 2 weeks, and maximal reduction is generally attained after 4 weeks. In long-term follow-up studies (without placebo control), the effect of valsartan appeared to be maintained for up to 2 years. The antihypertensive effect is independent of age, gender or race. The latter finding regarding race is based on pooled data and should be viewed with caution, because antihypertensive drugs that affect the renin- angiotensin system (that is, ACE inhibitors and angiotensin-II blockers) have generally been found to be less effective in low-renin hypertensives (frequently blacks) than in high-renin hypertensives (frequently whites). In pooled, randomized, controlled trials of Valsartan that included a total of 140 blacks and 830 whites, valsartan and an ACE-inhibitor control were generally at least as effective in blacks as whites. The explanation for this difference from previous findings is unclear.
Abrupt withdrawal of valsartan has not been associated with a rapid increase in blood pressure.
The blood pressure-lowering effect of valsartan and thiazide-type diuretics are approximately additive.
The 7 studies of valsartan monotherapy included over 2,000 patients randomized to various doses of valsartan and about 800 patients randomized to placebo. Doses below 80 mg were not consistently distinguished from those of placebo at trough, but doses of 80, 160 and 320 mg produced dose-related decreases in systolic and diastolic blood pressure, with the difference from placebo of approximately 6-9/3-5 mmHg at 80 to 160 mg and 9/6 mmHg at 320 mg. In a controlled trial the addition of HCTZ to valsartan 80 mg resulted in additional lowering of systolic and diastolic blood pressure by approximately 6/3 and 12/5 mmHg for 12.5 and 25 mg of HCTZ, respectively, compared to valsartan 80 mg alone.
Patients with an inadequate response to 80 mg once daily were titrated to either 160 mg once daily or 80 mg twice daily, which resulted in a similar response in both groups.
In controlled trials, the antihypertensive effect of once-daily valsartan 80 mg was similar to that of once-daily enalapril 20 mg or once-daily lisinopril 10 mg.
There are no trials of Valsartan demonstrating reductions in cardiovascular risk in patients with hypertension, but at least one pharmacologically similar drug has demonstrated such benefits.
There was essentially no change in heart rate in valsartan-treated patients in controlled trials.
Pediatric Hypertension
Children Between 6 to 16 Years of Age
In a clinical study involving 261 hypertensive pediatric patients 6 to 16 years of age, patients who weighed less than 35 kg received 10, 40 or 80 mg of valsartan daily (low, medium and high doses), and patients who weighed greater than or equal to 35 kg received 20, 80, and 160 mg of valsartan daily (low, medium and high doses). Renal and urinary disorders, and essential hypertension with or without obesity were the most common underlying causes of hypertension in children enrolled in this study. At the end of 2 weeks, valsartan reduced both systolic and diastolic blood pressure in a dose- dependent manner. Overall, the three dose levels of valsartan (low, medium and high) significantly reduced systolic blood pressure by 8, 10, and 12 mm Hg from the baseline, respectively. Patients were re-randomized to either continue receiving the same dose of valsartan or were switched to placebo. In patients who continued to receive the medium and high doses of valsartan, systolic blood pressure at trough was 4 and 7 mm Hg lower than patients who received the placebo treatment. In patients receiving the low dose of valsartan, systolic blood pressure at trough was similar to that of patients who received the placebo treatment. Overall, the dose-dependent antihypertensive effect of valsartan was consistent across all the demographic subgroups.
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's Diovan (valsartan) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information.
14.2 Heart Failure
The Valsartan Heart Failure Trial (Val-HeFT) was a multinational, double-blind study in which 5,010 patients with NYHA class II (62%) to IV (2%) heart failure and LVEF less than 40%, on baseline therapy chosen by their physicians, were randomized to placebo or valsartan (titrated from 40 mg twice daily to the highest tolerated dose or 160 mg twice daily) and followed for a mean of about 2 years. Although Val-HeFT's primary goal was to examine the effect of valsartan when added to an ACE inhibitor, about 7% were not receiving an ACE inhibitor. Other background therapy included diuretics (86%), digoxin (67%), and beta-blockers (36%). The population studied was 80% male, 46% 65 years or older and 89% Caucasian. At the end of the trial, patients in the valsartan group had a blood pressure that was 4 mmHg systolic and 2 mmHg diastolic lower than the placebo group. There were two primary end points, both assessed as time to first event: all-cause mortality and heart failure morbidity, the latter defined as all-cause mortality, sudden death with resuscitation, hospitalization for heart failure, and the need for intravenous inotropic or vasodilatory drugs for at least 4 hours. These results are summarized in the following table.
|
** Placebo** |
** Valsartan** |
** Hazard Ratio** |
** Nominal** | |
|
All-cause mortality |
484 |
495 |
1.02 |
0.8 |
|
HF morbidity |
801 |
723 |
0.87 |
0.009 |
|
*CI = Confidence Interval |
Although the overall morbidity result favored valsartan, this result was largely driven by the 7% of patients not receiving an ACE inhibitor, as shown in the following table.
|
** Without ACE Inhibitor** |
** With ACE Inhibitor** | |||
|
** Placebo** |
** Valsartan** |
** Placebo** |
** Valsartan** | |
|
Events (%) |
77 (42.5%) |
46 (24.9%) |
724 (31.2%) |
677 (29.1%) |
|
Hazard ratio (95% CI) |
0.51 (0.35, 0.73) |
0.92 (0.82, 1.02) | ||
|
p-value |
0.0002 |
0.0965 |
The modest favorable trend in the group receiving an ACE inhibitor was largely driven by the patients receiving less than the recommended dose of ACE inhibitor. Thus, there is little evidence of further clinical benefit when valsartan is added to an adequate dose of ACE inhibitor.
Secondary end points in the subgroup not receiving ACE inhibitors were as follows.
|
** Placebo** |
** Valsartan** |
** Hazard Ratio** | |
|
Components of HF morbidity | |||
|
All-cause mortality |
49 (27.1%) |
32 (17.3%) |
0.59 (0.37, 0.91) |
|
Sudden death with resuscitation |
2 (1.1%) |
1 (0.5%) |
0.47 (0.04, 5.20) |
|
CHF therapy |
1 (0.6%) |
0 (0.0%) |
– |
|
CHF hospitalization |
48 (26.5%) |
24 (13.0%) |
0.43 (0.27, 0.71) |
|
Cardiovascular mortality |
40 (22.1%) |
29 (15.7%) |
0.65 (0.40, 1.05) |
|
Non-fatal morbidity |
49 (27.1%) |
24 (13.0%) |
0.42 (0.26, 0.69) |
In patients not receiving an ACE inhibitor, valsartan-treated patients had an increase in ejection fraction and reduction in left ventricular internal diastolic diameter (LVIDD).
Effects were generally consistent across subgroups defined by age and gender for the population of patients not receiving an ACE inhibitor. The number of black patients was small and does not permit a meaningful assessment in this subset of patients.
14.3 Post-Myocardial Infarction
The VALsartan In Acute myocardial iNfarcTion trial (VALIANT) was a randomized, controlled, multinational, double-blind study in 14,703 patients with acute myocardial infarction and either heart failure (signs, symptoms or radiological evidence) or left ventricular systolic dysfunction (ejection fraction ≤40% by radionuclide ventriculography or ≤35% by echocardiography or ventricular contrast angiography). Patients were randomized within 12 hours to 10 days after the onset of myocardial infarction symptoms to one of three treatment groups: valsartan (titrated from 20 or 40 mg twice daily to the highest tolerated dose up to a maximum of 160 mg twice daily), the ACE inhibitor, captopril (titrated from 6.25 mg three times daily to the highest tolerated dose up to a maximum of 50 mg three times daily), or the combination of valsartan plus captopril. In the combination group, the dose of valsartan was titrated from 20 mg twice daily to the highest tolerated dose up to a maximum of 80 mg twice daily; the dose of captopril was the same as for monotherapy. The population studied was 69% male, 94% Caucasian, and 53% were 65 years of age or older. Baseline therapy included aspirin (91%), beta- blockers (70%), ACE inhibitors (40%), thrombolytics (35%) and statins (34%). The mean treatment duration was 2 years. The mean daily dose of Valsartan in the monotherapy group was 217 mg.
The primary endpoint was time to all-cause mortality. Secondary endpoints included (1) time to cardiovascular (CV) mortality, and (2) time to the first event of cardiovascular mortality, reinfarction, or hospitalization for heart failure.
The results are summarized in the following table.
|
** Valsartan vs. Captopril (N=4,909) (N=4,909)** |
** Valsartan + Captopril vs. Captopril (N=4,885) (N=4,909)** | |||||
|
** No. of Deaths Valsartan/ Captopril** |
** Hazard Ratio CI** |
** p-value** |
** No. of Deaths Comb/ Captopril** |
** Hazard Ratio CI** |
** p-value** | |
|
All-cause mortality |
979 (19.9%) / 958 (19.5%) |
1.001 (0.902, 1.111) |
0.98 |
941 (19.3%) / 958 (19.5%) |
0.984 (0.886, 1.093) |
0.73 |
|
CV mortality |
827 (16.8%) / 830 (16.9%) |
0.976 (0.875, 1.090) | ||||
|
CV mortality, hospitalization for HF, and recurrent non-fatal MI |
1,529 (31.1%) / 1,567(31.9%) |
0.955 (0.881, 1.035) |
There was no difference in overall mortality among the three treatment groups. There was thus no evidence that combining the ACE inhibitor captopril and the angiotensin II blocker valsartan was of value.
The data were assessed to see whether the effectiveness of valsartan could be demonstrated by showing in a non-inferiority analysis that it preserved a fraction of the effect of captopril, a drug with a demonstrated survival effect in this setting. A conservative estimate of the effect of captopril (based on a pooled analysis of 3 post-infarction studies of captopril and 2 other ACE inhibitors) was a 14% to 16% reduction in mortality compared to placebo. Valsartan would be considered effective if it preserved a meaningful fraction of that effect and unequivocally preserved some of that effect. As shown in the table, the upper bound of the CI for the hazard ratio (valsartan/captopril) for overall or CV mortality is 1.09 to 1.11, a difference of about 9% to 11%, thus making it unlikely that valsartan has less than about half of the estimated effect of captopril and clearly demonstrating an effect of valsartan. The other secondary endpoints were consistent with this conclusion.
Effects on Mortality Amongst Subgroups in VALIANT
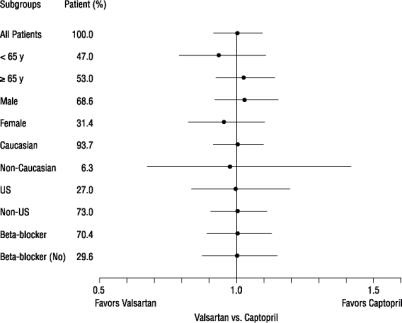
There were no clear differences in all-cause mortality based on age, gender, race, or baseline therapies, as shown in the figure above.
SPL UNCLASSIFIED SECTION
Store at 25°C (77°F); excursions permitted to 15°C -30°C (59°F - 86°F) [see USP Controlled Room Temperature].
Protect from moisture.
Dispense in tight container (USP).
SPL PATIENT PACKAGE INSERT SECTION
|
** PATIENT INFORMATION** |
|
** What is the most important information I should know about Valsartan
Tablets?**
|
|
** What is Valsartan Tablets?**
Valsartan Tablets should not be used to treat high blood pressure in children
less than 1 year of age. |
|
** Do not take VALSARTAN TABLETS if you:**
|
|
** Before taking Valsartan Tablets, tell your healthcare provider about all of your medical conditions including, if you:**
** Tell your healthcare provider about all the medicines you take** including
prescription and over- the-counter medicines, vitamins and herbal supplements.
Valsartan Tablets may affect the way other medicines work.
Know the medicines you take. Keep a list of your medicines with you to show to your healthcare provider and pharmacist when a new medicine is prescribed. Talk to your healthcare provider or pharmacist before you start taking any new medicine. |
|
** How should I take Valsartan Tablets?**
o your child is ≥ 6 years of age and cannot swallow tablets, or
o Shake the bottle of suspension well for at least 10 seconds before pouring the dose of medicine to give to your child *** For adults** with heart failure or who have had a heart attack, take Valsartan Tablets 2 times each day. Your healthcare provider may start you on a low dose of Valsartan Tablets and may increase the dose during your treatment.
If you take too much Valsartan Tablets, call your healthcare provider, or go to the nearest hospital emergency room. |
|
** What are the possible side effects of Valsartan Tablets?** *** Valsartan Tablets can cause serious side effects, including: See "What is the most important information I should know about Valsartan Tablets?"** *** Low blood pressure (hypotension).** Low blood pressure can happen with Valsartan Tablets, especially when you first start taking it and can cause you to feel lightheaded. Feeling lightheaded is most likely to happen if you:
Lie down, if you feel lightheaded, dizzy or faint. Call your healthcare provider right away. *** Kidney problems.** Kidney problems may get worse in people that already have kidney disease or heart problems. Your doctor may do blood tests to check for this. *** Increased potassium in your blood.** Some people may develop increased potassium in the blood during treatment with Valsartan Tablets. Your doctor may do a blood test to check your potassium levels as needed. ** The most common side effects of Valsartan Tablets when used to treat people with high blood pressure include:**
** The most common side effects of Valsartan Tablets when used to treat people with heart failure include:**
** The most common side effects of Valsartan Tablets when used to treat people after a heart attack that cause them to stop taking Valsartan Tablets include: **
You should not stop taking Valsartan Tablets without talking to your
healthcare provider. These are not all of the possible side effects of
Valsartan Tablets. For more information, ask your healthcare provider or
pharmacist. |
|
** How should I store Valsartan Tablets?**
** Keep Valsartan Tablets and all medicines out of the reach of children.** |
|
** General information about the safe and effective use of Valsartan Tablets.
** |
|
** What are the ingredients in Valsartan Tablets?** |
Additional pediatric use information is approved for Novartis Pharmaceuticals Corporation's Diovan (valsartan) tablets. However, due to Novartis Pharmaceuticals Corporation's marketing exclusivity rights, this drug product is not labeled with that information.
This Patient Information has been approved by the U.S. Food and Drug Administration Revised: 6/2022
