Aptiom
These highlights do not include all the information needed to use APTIOM safely and effectively. See full prescribing information for APTIOM. APTIOM (eslicarbazepine acetate) tablets, for oral useInitial U.S. Approval: 2013
3d0c9554-eaeb-4694-8089-00133fcadce3
HUMAN PRESCRIPTION DRUG LABEL
Nov 10, 2023
Sumitomo Pharma America, Inc.
DUNS: 131661746
Products 5
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ESLICARBAZEPINE ACETATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ESLICARBAZEPINE ACETATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ESLICARBAZEPINE ACETATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
ESLICARBAZEPINE ACETATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
ESLICARBAZEPINE ACETATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (4)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL – TITRATION SAMPLE PACK CARTON
NDC 63402-248-56
ONCE DAILY
Aptiom®
(eslicarbazepine acetate) Tablets
400 mg and 800 mg
ATTENTION DISPENSER: Dispense an enclosed
Medication Guide to each patient.
Rx Only
This package contains 56 tablets in 4 titration sample packs.
Each titration sample pack contains 14 tablets
(7 tablets of 400 mg and 7 tablets of 800 mg).
Please see the enclosed full Prescribing Information.
400 mg
per tablet and
800 mg
per tablet
Titration Sample Pack
Professional Samples
Not for Sale or
Reimbursement

INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
APTIOM is indicated for the treatment of partial-onset seizures in patients 4 years of age and older.
APTIOM is indicated for the treatment of partial-onset seizures in patients 4 years of age and older. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
APTIOM is contraindicated in patients with a hypersensitivity to eslicarbazepine acetate or oxcarbazepine [see Warnings and Precautions (5.2, 5.3, and 5.4)].
Hypersensitivity to eslicarbazepine acetate or oxcarbazepine. (4)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Behavior and Ideation
Antiepileptic drugs (AEDs), including APTIOM, increase the risk of suicidal thoughts or behavior in patients taking these drugs for any indication. Patients treated with any AED for any indication should be monitored for the emergence or worsening of depression, suicidal thoughts or behavior, and/or any unusual changes in mood or behavior.
Pooled analyses of 199 placebo-controlled clinical trials (mono- and adjunctive therapy) of 11 different AEDs showed that patients randomized to one of the AEDs had approximately twice the risk (adjusted Relative Risk 1.8, 95% confidence interval [CI]: 1.2, 2.7) of suicidal thinking or behavior compared to patients randomized to placebo. In these trials, which had a median treatment duration of 12 weeks, the estimated incidence of suicidal behavior or ideation among 27,863 AED-treated patients was 0.43%, compared to 0.24% among 16,029 placebo-treated patients, representing an increase of approximately one case of suicidal thinking or behavior for every 530 patients treated. There were four suicides in drug-treated patients in the trials and none in placebo-treated patients, but the number of events is too small to allow any conclusion about drug effect on suicide.
The increased risk of suicidal thoughts or behavior with AEDs was observed as early as one week after starting treatment with AEDs and persisted for the duration of treatment assessed. Because most trials included in the analysis did not extend beyond 24 weeks, the risk of suicidal thoughts or behavior beyond 24 weeks could not be assessed.
The risk of suicidal thoughts or behavior was generally consistent among drugs in the data analyzed. The finding of increased risk with AEDs of varying mechanisms of action and across a range of indications suggests that the risk applies to all AEDs used for any indication. The risk did not vary substantially by age (5-100 years) in the clinical trials analyzed.
Table 3 shows absolute and relative risk by indication for all evaluated AEDs.
Table 3: Risk of Suicidal Thoughts or Behaviors by Indication for Antiepileptic Drugs in the Pooled Analysis|
Indication |
Placebo Patients with Events Per 1000 Patients |
Drug Patients with Events Per 1000 Patients |
Relative Risk: Incidence of Events in Drug Patients/Incidence in Placebo Patients |
Risk Differences: Additional Drug Patients with Events Per 1000 Patients |
|
Epilepsy |
1.0 |
3.4 |
3.5 |
2.4 |
|
Psychiatric |
5.7 |
8.5 |
1.5 |
2.9 |
|
Other |
1.0 |
1.8 |
1.9 |
0.9 |
|
Total |
2.4 |
4.3 |
1.8 |
1.9 |
The relative risk for suicidal thoughts or behavior was higher in clinical trials in patients with epilepsy than in clinical trials in patients with psychiatric or other conditions, but the absolute risk differences were similar for epilepsy and psychiatric indications.
Anyone considering prescribing APTIOM or any other AED must balance this risk with the risk of untreated illness. Epilepsy and many other illnesses for which AEDs are prescribed are themselves associated with morbidity and mortality and an increased risk of suicidal thoughts and behavior. Should suicidal thoughts and behavior emerge during treatment, the prescriber needs to consider whether the emergence of these symptoms in any given patient may be related to the illness being treated.
Patients, their caregivers, and families should be informed that AEDs increase the risk of suicidal thoughts and behavior and should be advised of the need to be alert for the emergence or worsening of the signs and symptoms of depression; any unusual changes in mood or behavior; or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Behaviors of concern should be reported immediately to healthcare providers.
5.2 Serious Dermatologic Reactions
Serious dermatologic reactions including Stevens-Johnson Syndrome (SJS) and toxic epidermal necrolysis (TEN) have been reported in association with APTIOM use. Serious and sometimes fatal dermatologic reactions, including TEN and SJS, have also been reported in patients using oxcarbazepine or carbamazepine which are chemically related to APTIOM. The reporting rate of these reactions associated with oxcarbazepine use exceeds the background incidence rate estimates by a factor of 3- to 10-fold. The reporting rates for Aptiom have not been determined.
Risk factors for the development of serious and potentially fatal dermatologic reactions with APTIOM use have not been identified.
If a patient develops a dermatologic reaction while taking APTIOM, discontinue APTIOM use, unless the reaction is clearly not drug-related. Patients with a prior dermatologic reaction with oxcarbazepine, carbamazepine, or APTIOM should ordinarily not be treated with APTIOM [see Contraindications (4)].
5.3 Drug Reaction with Eosinophilia and Systemic Symptoms
(DRESS)/Multiorgan Hypersensitivity
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), also known as Multiorgan Hypersensitivity, has been reported in patients taking APTIOM. DRESS may be fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, and/or lymphadenopathy, in association with other organ system involvement, such as hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis sometimes resembling an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its expression, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, the patient should be evaluated immediately. APTIOM should be discontinued and not be resumed if an alternative etiology for the signs or symptoms cannot be established. Patients with a prior DRESS reaction with either oxcarbazepine or APTIOM should not be treated with APTIOM [see Contraindications (4)].
5.4 Anaphylactic Reactions and Angioedema
Rare cases of anaphylaxis and angioedema have been reported in patients taking APTIOM. Anaphylaxis and angioedema associated with laryngeal edema can be fatal. If a patient develops any of these reactions after treatment with APTIOM, the drug should be discontinued. Patients with a prior anaphylactic- type reaction with either oxcarbazepine or APTIOM should not be treated with APTIOM [see Contraindications (4)].
5.5 Hyponatremia
Clinically significant hyponatremia (sodium <125 mEq/L) can develop in patients taking APTIOM.
Measurement of serum sodium and chloride levels should be considered during maintenance treatment with APTIOM, particularly if the patient is receiving other medications known to decrease serum sodium levels, and should be performed if symptoms of hyponatremia develop (e.g., nausea/vomiting, malaise, headache, lethargy, confusion, irritability, muscle weakness/spasms, obtundation, or increase in seizure frequency or severity). Cases of symptomatic hyponatremia and syndrome of inappropriate antidiuretic hormone secretion (SIADH) have been reported during postmarketing use. In clinical trials, patients whose treatment with APTIOM was discontinued because of hyponatremia generally experienced normalization of serum sodium within a few days without additional treatment.
In the controlled adult adjunctive epilepsy trials, 4/415 patients (1.0%) treated with 800 mg and 6/410 (1.5%) patients treated with 1200 mg of APTIOM had at least one serum sodium value less than 125 mEq/L, compared to none of the patients assigned to placebo. A higher percentage of APTIOM-treated patients (5.1%) than placebo-treated patients (0.7%) experienced decreases in sodium values of more than 10 mEq/L. These effects were dose-related and generally appeared within the first 8 weeks of treatment (as early as after 3 days). Serious, life-threatening complications were reported with APTIOM- associated hyponatremia (as low as 112 mEq/L) including seizures, severe nausea/vomiting leading to dehydration, severe gait instability, and injury. Some patients required hospitalization and discontinuation of APTIOM. Concurrent hypochloremia was also present in patients with hyponatremia. Hyponatremia was also observed in adult monotherapy trials and in pediatric trials. Depending on the severity of hyponatremia, the dose of APTIOM may need to be reduced or discontinued.
5.6 Neurological Adverse Reactions
Dizziness and Disturbance in Gait and Coordination
APTIOM causes dose-related increases in adverse reactions related to dizziness and disturbance in gait and coordination (dizziness, ataxia, vertigo, balance disorder, gait disturbance, nystagmus, and abnormal coordination) [see Adverse Reactions (6.1)]. In controlled adult adjunctive epilepsy trials, these events were reported in 26% and 38% of patients randomized to receive APTIOM at doses of 800 mg and 1200 mg/day, respectively, compared to 12% of placebo-treated patients. Events related to dizziness and disturbance in gait and coordination were more often serious in APTIOM-treated patients than in placebo-treated patients (2% vs. 0%), and more often led to study withdrawal in APTIOM-treated patients than in placebo-treated patients (9% vs. 0.7%). There was an increased risk of these adverse reactions during the titration period (compared to the maintenance period) and there also may be an increased risk of these adverse reactions in patients 60 years of age and older compared to younger adults. Nausea and vomiting also occurred with these events. Adverse reactions related to dizziness and disturbance in gait and coordination were also observed in adult monotherapy trials and pediatric trials.
The incidence of dizziness was greater with the concomitant use of APTIOM and carbamazepine compared to the use of APTIOM without carbamazepine in adult and pediatric trials. Therefore, consider dosage modifications of both APTIOM and carbamazepine if these drugs are used concomitantly [see Dosage and Administration (2.3)].
Somnolence and Fatigue
APTIOM causes dose-dependent increases in somnolence and fatigue-related adverse reactions (fatigue, asthenia, malaise, hypersomnia, sedation, and lethargy). In the controlled adult adjunctive epilepsy trials, these events were reported in 13% of placebo patients, 16% of patients randomized to receive 800 mg/day APTIOM, and 28% of patients randomized to receive 1200 mg/day APTIOM. Somnolence and fatigue-related events were serious in 0.3% of APTIOM-treated patients (and 0 placebo patients) and led to discontinuation in 3% of APTIOM-treated patients (and 0.7% of placebo-treated patients). Somnolence and fatigue-related reactions were also observed in adult monotherapy trials and in pediatric trials.
Cognitive Dysfunction
APTIOM causes dose-dependent increases in cognitive dysfunction-related events in adults (memory impairment, disturbance in attention, amnesia, confusional state, aphasia, speech disorder, slowness of thought, disorientation, and psychomotor retardation). In the controlled adult adjunctive epilepsy trials, these events were reported in 1% of placebo patients, 4% of patients randomized to receive 800 mg/day APTIOM, and 7% of patients randomized to receive 1200 mg/day APTIOM. Cognitive dysfunction-related events were serious in 0.2% of APTIOM-treated patients (and 0.2% of placebo patients) and led to discontinuation in 1% of APTIOM-treated patients (and 0.5% of placebo-treated patients). Cognitive dysfunction events were also observed in adult monotherapy trials.
Visual Changes
APTIOM causes dose-dependent increases in events related to visual changes including diplopia, blurred vision, and impaired vision. In the controlled adult adjunctive epilepsy trials, these events were reported in 16% of patients randomized to receive APTIOM compared to 6% of placebo patients. Eye events were serious in 0.7% of APTIOM-treated patients (and 0 placebo patients) and led to discontinuation in 4% of APTIOM-treated patients (and 0.2% of placebo-treated patients). There was an increased risk of these adverse reactions during the titration period (compared to the maintenance period) and also in patients 60 years of age and older (compared to younger adults). The incidence of diplopia was greater with the concomitant use of APTIOM and carbamazepine compared to the use of APTIOM without carbamazepine (up to 16% vs. 6%, respectively) [see Dosage and Administration (2.3)]. Similar adverse reactions related to visual changes were also observed in adult monotherapy trials and in pediatric trials.
Hazardous Activities
Prescribers should advise patients against engaging in hazardous activities requiring mental alertness, such as operating motor vehicles or dangerous machinery, until the effect of APTIOM is known.
5.7 Withdrawal of AEDs
As with all antiepileptic drugs, APTIOM should be withdrawn gradually because of the risk of increased seizure frequency and status epilepticus, but if withdrawal is needed because of a serious adverse event, rapid discontinuation can be considered.
5.8 Drug Induced Liver Injury
Hepatic effects, ranging from mild to moderate elevations in transaminases (>3 times the upper limit of normal) to rare cases with concomitant elevations of total bilirubin (>2 times the upper limit of normal) have been reported with APTIOM use. Baseline evaluations of liver laboratory tests are recommended. The combination of transaminase elevations and elevated bilirubin without evidence of obstruction is generally recognized as an important predictor of severe liver injury. APTIOM should be discontinued in patients with jaundice or other evidence of significant liver injury (e.g., laboratory evidence).
5.9 Abnormal Thyroid Function Tests
Dose-dependent decreases in serum T3 and T4 (free and total) values have been observed in patients taking APTIOM. These changes were not associated with other abnormal thyroid function tests suggesting hypothyroidism. Abnormal thyroid function tests should be clinically evaluated.
5.10 Hematologic Adverse Reactions
Rare cases of pancytopenia, agranulocytosis, and leukopenia have been reported during postmarketing use in patients treated with APTIOM. Discontinuation of APTIOM should be considered in patients who develop pancytopenia, agranulocytosis, or leukopenia.
- Suicidal Behavior and Ideation: Monitor for suicidal thoughts or behavior. (5.1)
- Serious Dermatologic Reactions, Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS), Anaphylactic Reactions and Angioedema: Monitor and discontinue if another cause cannot be established. (5.2, 5.3, 5.4)
- Hyponatremia: Monitor sodium levels in patients at risk or patients experiencing hyponatremia symptoms. (5.5)
- Neurological Adverse Reactions: Monitor for dizziness, disturbance in gait and coordination, somnolence, fatigue, cognitive dysfunction, and visual changes. Use caution when driving or operating machinery. (5.6)
- Withdrawal of APTIOM: Withdraw APTIOM gradually to minimize the risk of increased seizure frequency and status epilepticus. (2.6, 5.7, 8.1)
- Drug Induced Liver Injury: Discontinue APTIOM in patients with jaundice or evidence of significant liver injury. (5.8)
- Hematologic Adverse Reactions: Consider discontinuing. (5.10)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are described in more detail in the Warnings and Precautions section of the label:
- Suicidal Behavior and Ideation [see Warnings and Precautions (5.1)]
- Serious Dermatologic Reactions [see Warnings and Precautions (5.2)]
- Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS)/Multiorgan Hypersensitivity [see Warnings and Precautions (5.3)]
- Anaphylactic Reactions and Angioedema [see Warnings and Precautions (5.4)]
- Hyponatremia [see Warnings and Precautions (5.5)]
- Neurological Adverse Reactions [see Warnings and Precautions (5.6)]
- Drug Induced Liver Injury [see Warnings and Precautions (5.8)]
- Abnormal Thyroid Function Tests [see Warnings and Precautions (5.9)]
- Pancytopenia, Agranulocytosis, and Leukopenia [see Warnings and Precautions (5.10)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adult Patients
In monotherapy trials in patients with partial-onset seizures [Study 1 and Study 2, see Clinical Studies (14.1)], 365 patients received APTIOM, of whom 225 were treated for longer than 12 months and 134 for longer than 24 months. Of the patients in those trials, 95% were between 18 and 65 years old; 48% were male, and 84% were Caucasian. Across controlled and uncontrolled trials in patients receiving adjunctive therapy for partial-onset seizures, 1195 patients received APTIOM, of whom 586 were treated for longer than 6 months and 462 for longer than 12 months. In the placebo controlled adjunctive therapy trials in patients with partial-onset seizures (Study 3, Study 4 and Study 5), 1021 patients received APTIOM. Of the patients in those trials, approximately 95% were between 18 and 60 years old, approximately 50% were male, and approximately 80% were Caucasian.
Monotherapy Historical Control Trials
In the monotherapy epilepsy trials (Study 1 and Study 2), 13% of patients randomized to receive APTIOM at the recommended doses of 1200 mg and 1600 mg once daily discontinued from the trials as a result of an adverse event. The adverse reaction most commonly (≥1% on APTIOM) leading to discontinuation was hyponatremia.
Adverse reactions observed in these studies were generally similar to those observed and attributed to drug in adjunctive placebo-controlled studies. Because these studies did not include a placebo control group, causality could not be established.
Dizziness, nausea, somnolence, and fatigue were all reported at lower incidences during the AED Withdrawal Phase and Monotherapy Phase compared with the Titration Phase.
Adjunctive Therapy Controlled Trials
In the controlled adjunctive therapy epilepsy trials (Study 3, Study 4, and Study 5), the rate of discontinuation as a result of any adverse reaction was 14% for the 800 mg dose, 25% for the 1200 mg dose, and 7% in subjects randomized to placebo. The adverse reactions most commonly (≥1% in any APTIOM treatment group, and greater than placebo) leading to discontinuation, in descending order of frequency, were dizziness, nausea, vomiting, ataxia, diplopia, somnolence, headache, blurred vision, vertigo, asthenia, fatigue, rash, dysarthria, and tremor.
The most frequently reported adverse reactions in patients receiving APTIOM at doses of 800 mg or 1200 mg (≥4% and ≥2% greater than placebo) were dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor.
Table 4 gives the incidence of adverse reactions that occurred in ≥2% of subjects with partial-onset seizures in any APTIOM treatment group and for which the incidence was greater than placebo during the controlled clinical trials. Adverse reactions during titration were less frequent for patients who began therapy at an initial dose of 400 mg for 1 week and then increased to 800 mg compared to patients who initiated therapy at 800 mg.
Table 4: Adverse Reactions Incidence in Pooled Controlled Clinical Trials of Adjunctive Therapy in Adults (Events ≥2% of Patients in the APTIOM 800 mg or 1200 mg Dose Group and More Frequent Than in the Placebo Group)|
Placebo |
APTIOM | ||
|---|---|---|---|
|
800 mg |
1200 mg | ||
|
(N=426) |
(N=415) |
(N=410) | |
|
Ear and labyrinth disorders |
<1 |
2 |
6 |
|
Eye disorders |
2 |
9 |
11 |
|
Gastrointestinal disorders |
5 |
10 |
16 |
|
General disorders and administration site conditions |
4 |
4 |
7 |
|
Infections and Infestations |
1 |
2 |
2 |
|
Injury, poisoning and procedural complications |
1 |
3 |
1 |
|
Metabolism and nutrition disorders |
<1 |
2 |
2 |
|
Nervous system disorders |
9 |
20 |
28 |
|
Psychiatric disorders |
2 |
1 |
3 |
|
Respiratory, thoracic and mediastinal disorders |
1 |
2 |
1 |
|
Skin and subcutaneous tissue disorders |
1 |
1 |
3 |
|
Vascular disorders |
1 |
1 |
2 |
Pediatric Patients (4 to 17 Years of Age)
Clinical studies of pediatric patients 4 to 17 years of age were conducted which support the safety and tolerability of APTIOM for the treatment of partial-onset seizures. Across studies in pediatric patients with partial- onset seizures, 393 patients ages 4 to 17 years received APTIOM, of whom 265 received APTIOM for at least 1 year. Adverse reactions reported in clinical studies of pediatric patients 4 to 17 years of age were similar to those seen in adult patients.
Other Adverse Reactions with APTIOM Use
Compared to placebo, APTIOM use was associated with slightly higher frequencies of decreases in hemoglobin and hematocrit, increases in total cholesterol, triglycerides, and LDL, and increases in creatine phosphokinase.
Adverse Reactions Based on Gender and Race
No significant gender differences were noted in the incidence of adverse reactions. Although there were few non-Caucasian patients, no differences in the incidences of adverse reactions compared to Caucasian patients were observed.
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of APTIOM. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Hematologic and Lymphatic Systems: leukopenia, agranulocytosis, thrombocytopenia, megaloblastic anemia, and pancytopenia [see Warnings and Precautions (5.10)]
Metabolism and Nutrition Disorders: syndrome of inappropriate antidiuretic hormone secretion (SIADH) [see Warnings and Precautions (5.5)]
- Most common adverse reactions in adult patients receiving APTIOM (≥4% and ≥2% greater than placebo): dizziness, somnolence, nausea, headache, diplopia, vomiting, fatigue, vertigo, ataxia, blurred vision, and tremor. (6.1)
- Adverse reactions in pediatric patients are similar to those seen in adult patients.
To report SUSPECTED ADVERSE REACTIONS, contact Sumitomo Pharma America, Inc. at 1-877-737-7226 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Other Antiepileptic Drugs
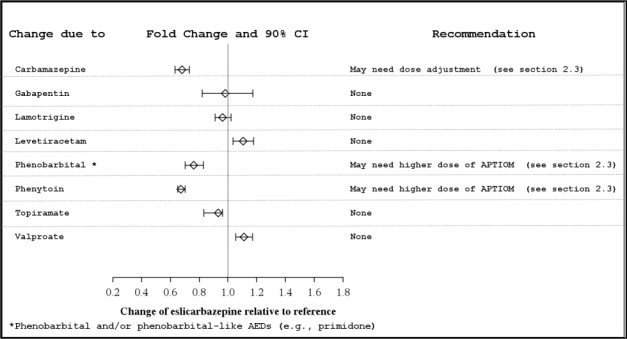
Several AEDs (e.g., carbamazepine, phenobarbital, phenytoin, and primidone) can induce enzymes that metabolize APTIOM and can cause decreased plasma concentrations of eslicarbazepine [see Clinical Pharmacology (12.3)]. Higher doses of Aptiom may be needed [see Dosage and Administration (2.4)].
7.2 CYP2C19 Substrates
APTIOM can inhibit CYP2C19, which can cause increased plasma concentrations of drugs that are metabolized by this isoenzyme (e.g., phenytoin, clobazam, and omeprazole) [see Clinical Pharmacology (12.3)]. Dose adjustment may be needed.
7.3 CYP3A4 Substrates
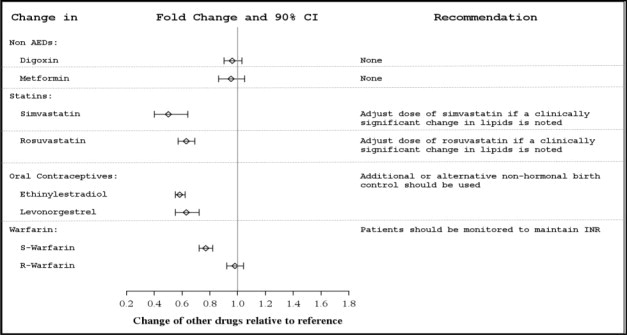
In vivo studies suggest that APTIOM can induce CYP3A4, decreasing plasma concentrations of drugs that are metabolized by this isoenzyme (e.g., simvastatin, lovastatin) [see Clinical Pharmacology (12.3)]. Dose adjustment of simvastatin and lovastatin may be needed if a clinically significant change in lipids is noted.
7.4 Oral Contraceptives
Because concomitant use of APTIOM and ethinylestradiol and levonorgestrel is associated with lower plasma levels of these hormones, females of reproductive potential should use additional or alternative non-hormonal birth control.
- Carbamazepine: May need dose adjustment for APTIOM or carbamazepine. (2.3, 5.6, 7.1)
- Phenytoin: Higher dosage of APTIOM may be necessary and dose adjustment may be needed for phenytoin. (2.3, 7.1, 7.2)
- Phenobarbital or Primidone: Higher dosage of APTIOM may be necessary. (2.3, 7.1)
- Hormonal Contraceptives: APTIOM may decrease the effectiveness of hormonal contraceptives. (7.4, 8.3)
DESCRIPTION SECTION
11 DESCRIPTION
The chemical name of APTIOM (eslicarbazepine acetate) is (S)-10-Acetoxy-10,11-dihydro-5H-dibenz[b,f]azepine-5-carboxamide. APTIOM is a dibenz[b,f]azepine-5-carboxamide derivative. Its molecular formula is C17H16N2O3 and its molecular weight is 296.32. The chemical structure is:
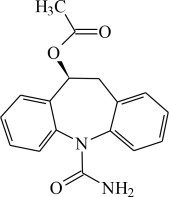
APTIOM is a white to off-white, odorless crystalline solid. It is insoluble in hexane, very slightly soluble in aqueous solvents and soluble in organic solvents such as acetone, acetonitrile, and methanol.
Each APTIOM tablet contains 200 mg, 400 mg, 600 mg or 800 mg of eslicarbazepine acetate and the following inactive ingredients: croscarmellose sodium, magnesium stearate, and povidone.
REFERENCES SECTION
15 REFERENCES
French JA, Wang S, Warnock B, Temkin N. Historical control monotherapy design in the treatment of epilepsy. Epilepsia 2010;51(10):1936-43.
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Instruct patients to administer APTIOM either as whole or as crushed tablets. Instruct patients to take APTIOM either with or without food. The APTIOM dosing regimen depends on age, weight, and renal function.
2.2 General Dosing Recommendations
Monotherapy and Adjunctive Therapy
Adult Patients
The recommended initial dosage of APTIOM is 400 mg administered orally once daily. For some patients, treatment may be initiated at 800 mg once daily if the need for seizure reduction outweighs an increased risk of adverse reactions during initiation [see Adverse Reactions (6.1)]. Dosage should be increased in weekly increments of 400 mg to 600 mg, based on clinical response and tolerability, to a recommended maintenance dosage of 800 mg to 1600 mg once daily. For patients on APTIOM monotherapy, the 800 mg once daily maintenance dose should generally be considered in patients who are unable to tolerate a 1200 mg daily dose. For patients on APTIOM adjunctive therapy, the 1600 mg daily dose should generally be considered in patients who did not achieve a satisfactory response with a 1200 mg daily dose.
Pediatric Patients (4 to 17 Years of Age)
In pediatric patients 4 to 17 years of age, the recommended dosing regimen is dependent upon body weight and is administered orally once daily. The recommended initial dosage of APTIOM is shown in Table 1. Dosage should be increased based on clinical response and tolerability, no more frequently than once per week. Titration increments should not exceed those shown in Table 1. The daily maintenance dosage should not exceed the maintenance dosage for each body weight range shown in Table 1.
Table 1: APTIOM Once Daily Dosage Schedule for Pediatric Patients 4 to 17 Years of Age|
Body Weight Range |
Initial and Maximum Titration Increment Dosage (mg/day) |
Maintenance Dosage (mg/day) |
|
11 to 21 kg |
200 |
400 to 600 |
|
22 to 31 kg |
300 |
500 to 800 |
|
32 to 38 kg |
300 |
600 to 900 |
|
more than |
400 |
800 to 1200 |
2.3 Dosage Modifications with Other Antiepileptic Drugs
Some adverse reactions occur more frequently when patients take APTIOM adjunctively with carbamazepine [see Warnings and Precautions (5.6)]. However, carbamazepine reduces the plasma concentration of eslicarbazepine [see Drug Interactions (7.1)]. When APTIOM and carbamazepine are taken concomitantly, the dose of APTIOM or carbamazepine may need to be adjusted based on efficacy and tolerability. For patients taking other enzyme-inducing AEDs (i.e., phenobarbital, phenytoin, and primidone), higher doses of APTIOM may be needed [see Drug Interactions (7.1)].
APTIOM should not be taken as an adjunctive therapy with oxcarbazepine.
2.4 Dosage Modifications in Patients with Renal Impairment
In patients with moderate and severe renal impairment (i.e., creatinine clearance < 50 mL/min), the initial, titration, and maintenance dosages should generally be reduced by 50%. Titration and maintenance dosages may be adjusted according to clinical response [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.5 Patients with Hepatic Impairment
Dose adjustments are not required in patients with mild to moderate hepatic impairment. Use of APTIOM in patients with severe hepatic impairment has not been studied, and use in these patients is not recommended [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.6 Discontinuation of APTIOM
When discontinuing APTIOM, reduce the dosage gradually and avoid abrupt discontinuation in order to minimize the risk of increased seizure frequency and status epilepticus [see Warnings and Precautions (5.7)].
- Adult Patients: The recommended initial dosage of APTIOM is 400 mg once daily. For some patients, treatment may be initiated at 800 mg once daily if the need for seizure reduction outweighs an increased risk of adverse reactions. Increase the dose in weekly increments of 400 mg to 600 mg once daily, based on clinical response and tolerability, to a recommended maintenance dosage of 800 mg to 1600 mg once daily. (2.2)
- Pediatric Patients: The recommended dosage of APTIOM is based on body weight and is administered orally once daily. Increase the dose in weekly intervals based on clinical response and tolerability, to the recommended maintenance dosage. (2.2)
- Patients with Moderate or Severe Renal Impairment: Reduce dosage by 50%. (2.4)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
APTIOM tablets are available in the following shapes and color (Table 2) with respective one-sided engraving:
Table 2: APTIOM Tablet Presentations|
Tablet Strength |
Tablet Color/Shape |
Tablet Markings |
Functional Score |
|
200 mg |
White oblong |
ESL 200 |
Yes |
|
400 mg |
White circular bi-convex |
ESL 400 |
No |
|
600 mg |
White oblong |
ESL 600 |
Yes |
|
800 mg |
White oblong |
ESL 800 |
Yes |
Tablets: 200 mg, 400 mg, 600 mg, 800 mg (3)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to AEDs, such as APTIOM, during pregnancy. Encourage women who are taking APTIOM during pregnancy to enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry by calling 1-888-233-2334 or visiting http://www.aedpregnancyregistry.org.
Risk Summary
Limited available data with APTIOM use in pregnant women are insufficient to inform a drug-associated risk of adverse developmental outcomes. In oral studies conducted in pregnant mice, rats, and rabbits, eslicarbazepine acetate demonstrated developmental toxicity, including increased incidence of malformations (mice), embryolethality (rats), and fetal growth retardation (all species), at clinically relevant doses (see Data). Advise a pregnant woman of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively. The background risk of major birth defects and miscarriage for the indicated population is unknown.
Data
Animal Data
When eslicarbazepine acetate was orally administered (150, 350, 650 mg/kg/day) to pregnant mice throughout organogenesis, increased incidences of fetal malformations was observed at all doses and fetal growth retardation was observed at the mid and high doses. A no-effect dose for adverse developmental effects was not identified. At the lowest dose tested, plasma eslicarbazepine exposure (Cmax, AUC) is less than that in humans at the maximum recommended human dose (MRHD, 1600 mg/day).
Oral administration of eslicarbazepine acetate (40, 160, 320 mg/kg/day) to pregnant rabbits throughout organogenesis resulted in fetal growth retardation and increased incidences of skeletal variations at the mid and high doses. The no-effect dose (40 mg/kg/day) is less than the MRHD on a mg/m2 basis.
Oral administration to pregnant rats (65, 125, 250 mg/kg/day) throughout organogenesis resulted in embryolethality at all doses, increased incidences of skeletal variations at the mid and high doses, and fetal growth retardation at the high dose. The lowest dose tested (65 mg/kg/day) is less than the MRHD on a mg/m2 basis.
When eslicarbazepine acetate was orally administered to female mice during pregnancy and lactation (150, 350, 650 mg/kg/day), the gestation period was prolonged at the highest dose tested. In offspring, a persistent reduction in offspring body weight and delayed physical development and sexual maturation were observed at the mid and high doses. The lowest dose tested (150 mg/kg/day) is less than the MRHD on a mg/m2 basis.
When eslicarbazepine acetate was orally administered (65, 125, 250 mg/kg/day) to rats during pregnancy and lactation, reduced offspring body weight was seen at the mid and high doses. Delayed sexual maturation and a neurological deficit (decreased motor coordination) were observed at the highest dose tested. The no-effect dose for adverse developmental effects (65 mg/kg/day) is less than the MRHD on a mg/m2 basis.
The rat data are of uncertain relevance to humans because of differences in metabolic profile between species.
8.2 Lactation
Eslicarbazepine is present in human milk. The effects of APTIOM on the breastfed infant or on milk production are unknown. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for APTIOM and any potential adverse effects on the breastfed infant from APTIOM or from the underlying maternal condition.
8.3 Females and Males of Reproductive Potential
Contraception
Use of APTIOM with hormonal contraceptives containing ethinylestradiol or levonorgestrel is associated with lower plasma levels of these hormones. Advise women of reproductive potential taking APTIOM who are using a contraceptive containing ethinylestradiol or levonorgestrel to use additional or alternative non-hormonal birth control [see Drug Interactions (7.4)].
Infertility
Eslicarbazepine acetate was evaluated in rats and mice for potential adverse impact on fertility of the parental and first generation [see Nonclinical Toxicology (13.1)]. In a fertility study in male and female mice, adverse developmental outcomes were observed in embryos. In a fertility study in male and female rats, impairment of female fertility by eslicarbazepine acetate was shown.
8.4 Pediatric Use
Safety and effectiveness of APTIOM have been established in the age groups 4 to 17 years. Use of APTIOM in these age groups is supported by evidence from adequate and well-controlled studies of APTIOM in adults with partial-onset seizures, pharmacokinetic data from adult and pediatric patients, and safety data from clinical studies in 393 pediatric patients 4 to 17 years of age [see Adverse Reactions (6.1) and Clinical Pharmacology (12.3)].
Safety and effectiveness in pediatric patients below the age of 4 years have not been established.
Animal Data
In a juvenile animal study in which eslicarbazepine acetate (40, 80, 160 mg/kg/day) was orally administered to young dogs for 10 months starting on postnatal day 21, adverse effects on bone growth (decreased bone mineral content and density) were seen in females at all doses at the end of the dosing period, but not at the end of a 2-month recovery period. Convulsions were seen at the highest dose tested. A no-effect dose for adverse effects in juvenile dogs was not identified. The lowest dose tested is less than the maximum recommended pediatric dose (1200 mg/day) on a body surface area (mg/m2) basis.
A separate juvenile animal study was conducted to assess possible adverse effects on the immune system. Eslicarbazepine acetate (10, 40, 80 mg/kg/day) was orally administered to young dogs for 17 weeks starting on postnatal day 21. No effects on the immune system were observed.
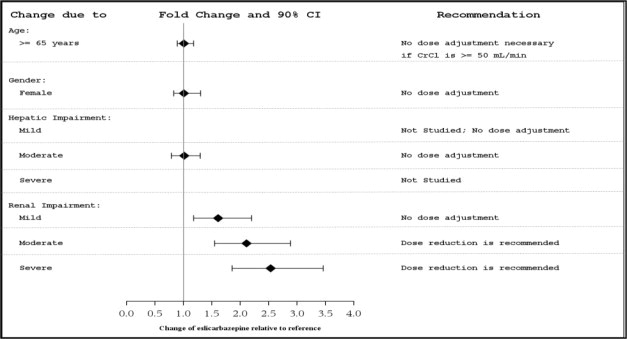
8.5 Geriatric Use
There were insufficient numbers of patients ≥65 years old enrolled in the controlled adjunctive epilepsy trials (N=15) to determine the efficacy of APTIOM in this patient population. The pharmacokinetics of APTIOM were evaluated in elderly healthy subjects (N=12) (Figure 1). Although the pharmacokinetics of eslicarbazepine are not affected by age independently, dose selection should take in consideration the greater frequency of renal impairment and other concomitant medical conditions and drug therapies in the elderly patient. Dose adjustment is necessary if CrCl is <50 mL/min [see Clinical Pharmacology (12.3)].
8.6 Patients with Renal Impairment
Clearance of eslicarbazepine is decreased in patients with impaired renal function and is correlated with creatinine clearance. Dosage adjustment is necessary in patients with CrCl<50 mL/min (Figure 1) [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
8.7 Patients with Hepatic Impairment
Dose adjustments are not required in patients with mild to moderate hepatic impairment (Figure 1). Use of APTIOM in patients with severe hepatic impairment has not been evaluated, and use in these patients is not recommended [see Clinical Pharmacology (12.3)].
Pregnancy: Based on animal data, may cause fetal harm. (8.1)
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
APTIOM is not a controlled substance.
9.2 Abuse
Prescription drug abuse is the intentional non-therapeutic use of a drug, even once, for its rewarding psychological or physiological effects. Drug addiction, which develops after repeated drug abuse, is characterized by a strong desire to take a drug despite harmful consequences, difficulty in controlling its use, giving a higher priority to drug use than to obligations, increased tolerance, and sometimes physical withdrawal. Drug abuse and drug addiction are separate and distinct from physical dependence (for example, abuse may not be accompanied by physical dependence) [see Drug Abuse and Dependence (9.3)].
In a human abuse study in recreational sedative abusers APTIOM showed no evidence of abuse. In Phase 1, 1.5% of the healthy volunteers taking APTIOM reported euphoria compared to 0.4% taking placebo.
9.3 Dependence
Physical dependence is characterized by withdrawal symptoms after abrupt discontinuation or a significant dose reduction of a drug.
There was some evidence of physical dependence or a withdrawal syndrome with APTIOM in a physical dependence study conducted in healthy volunteers who were maintained at a daily dose of 800 mg APTIOM for 4 weeks prior to discontinuation. The primary endpoint was the maximum change from steady-state baseline in the total score of the Physician's Withdrawal Checklist (PWC-34) during the 21-day discontinuation period. APTIOM and placebo were shown to be equivalent on the primary endpoint. Two out of 8 secondary endpoints (visual analog scales for anxiety and nausea) showed some increase in these symptoms for subjects who were maintained on APTIOM and discontinued, versus subjects who were maintained on placebo. In general, AEDs should not be abruptly discontinued in patients with epilepsy because of the risk of increased seizure frequency and status epilepticus.
OVERDOSAGE SECTION
10 OVERDOSAGE
10.1 Signs, Symptoms, and Laboratory Findings of Acute Overdose in Humans
Symptoms of overdose are consistent with the known adverse reactions of APTIOM and include hyponatremia (sometimes severe), dizziness, nausea, vomiting, somnolence, euphoria, oral paraesthesia, ataxia, walking difficulties, and diplopia. The maximum dosage studied in open-label adult monotherapy treatment following withdrawal of concomitant AEDs was 2400 mg once daily.
10.2 Treatment or Management of Overdose
There is no specific antidote for overdose with APTIOM. Symptomatic and supportive treatment should be administered as appropriate. Removal of the drug by gastric lavage and/or inactivation by administering activated charcoal should be considered.
Standard hemodialysis procedures result in partial clearance of APTIOM. Hemodialysis may be considered based on the patient's clinical state or in patients with significant renal impairment.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
APTIOM is extensively converted to eslicarbazepine, which is considered to be responsible for therapeutic effects in humans. The precise mechanism(s) by which eslicarbazepine exerts anticonvulsant activity is unknown but is thought to involve inhibition of voltage-gated sodium channels.
12.2 Pharmacodynamics
The effect of APTIOM on cardiac repolarization was evaluated in a randomized, double-blind, placebo- and active-controlled 4-period crossover trial in healthy adult men and women. Subjects received APTIOM 1200 mg once daily × 5 days, APTIOM 2400 mg once daily × 5 days, an active-control, moxifloxacin 400 mg × 1 dose on Day 5, and placebo once daily × 5 days. At both doses of APTIOM, no significant effect on the QTc interval was detected.
12.3 Pharmacokinetics
The pharmacokinetics of eslicarbazepine is linear and dose-proportional in the dose range of 400 mg to 1600 mg once daily, both in healthy adult subjects and patients. The apparent half-life of eslicarbazepine in plasma was 13-20 hours in adult epilepsy patients. Steady-state plasma concentrations are attained after 4 to 5 days of once daily dosing.
Absorption, Distribution, Metabolism, and Excretion
Absorption
APTIOM is mostly undetectable (0.01% of the systemic exposure) after oral administration. Eslicarbazepine, the major metabolite, is primarily responsible for the pharmacological effect of APTIOM. Peak plasma concentrations (Cmax) of eslicarbazepine are attained at 1-4 hours post-dose. Eslicarbazepine is highly bioavailable, because the amount of eslicarbazepine and glucuronide metabolites recovered in urine corresponded to more than 90% of an APTIOM dose. Food has no effect on the pharmacokinetics of eslicarbazepine after oral administration of APTIOM.
Distribution
The binding of eslicarbazepine to plasma proteins is relatively low (<40%) and independent of concentration. In vitro studies have shown that plasma protein binding was not relevantly affected by the presence of warfarin, diazepam, digoxin, phenytoin, or tolbutamide. Similarly, the binding of warfarin, diazepam, digoxin, phenytoin or tolbutamide was not significantly affected by the presence of eslicarbazepine. The apparent volume of distribution of eslicarbazepine is 61 L for body weight of 70 kg based on population PK analysis.
Metabolism
APTIOM is rapidly and extensively metabolized to its major active metabolite eslicarbazepine by hydrolytic first-pass metabolism. Eslicarbazepine corresponds to 91% of systemic exposure. The systemic exposure to minor active metabolites of (R)-licarbazepine is 5% and oxcarbazepine is 1%. The inactive glucuronides of these active metabolites correspond to approximately 3% of systemic exposure.
In in vitro studies in human liver microsomes, eslicarbazepine had no clinically relevant inhibitory effect on the activity of CYP1A2, CYP2A6, CYP2B6, CYP2D6, CYP2E1, and CYP3A4, and only a moderate inhibitory effect on CYP2C19. Studies with eslicarbazepine in fresh human hepatocytes showed no induction of enzymes involved in glucuronidation and sulfation of 7-hydroxy- coumarin. A mild activation of UGT1A1-mediated glucuronidation was observed in human hepatic microsomes.
No apparent autoinduction of metabolism has been observed with APTIOM in humans.
Excretion
APTIOM metabolites are eliminated from the systemic circulation primarily by renal excretion, in the unchanged and glucuronide conjugate forms. In total, eslicarbazepine and its glucuronide account for more than 90% of total metabolites excreted in urine, approximately two thirds in the unchanged form and one third as glucuronide conjugate. Other minor metabolites account for the remaining 10% excreted in the urine. In healthy subjects with normal renal function, the renal clearance of eslicarbazepine (approximately 20 mL/min) is substantially lower than glomerular filtration rate (80-120 mL/min), suggesting that renal tubular reabsorption occurs. The apparent plasma half- life of eslicarbazepine was 13-20 hours in epilepsy patients [see Dosage and Administration (2.4) and Use in Specific Populations (8.6)].
Specific Populations
Geriatric Patients (≥65 Years of Age)
The pharmacokinetic profile of eslicarbazepine was unaffected in elderly subjects with creatinine clearance >60 mL/min compared to healthy subjects (18-40 years) after single and repeated doses of 600 mg APTIOM during 8 days of dosing. No dose adjustment is necessary in adults based on age, if CrCl is ≥50 mL/min.
Pediatric Patients (4 to 17 Years of Age)
A pharmacokinetic study of APTIOM was performed in 29 pediatric patients with partial-onset seizures. Limited pharmacokinetic sampling was also performed during controlled pediatric adjunctive therapy partial-onset seizure studies. As in adult patients, APTIOM is rapidly and extensively metabolized to its major active metabolite eslicarbazepine. The pharmacokinetics of eslicarbazepine is linear and dose-proportional in the dose range of 5 to 30 mg/kg/day. Peak plasma concentrations (Cmax) of eslicarbazepine are attained at 1-3 hours post-dose.
A population pharmacokinetic analysis showed that body weight significantly correlates with the clearance of eslicarbazepine in pediatric patients; clearance increased with an increase in body weight. A weight-based dosing regimen is necessary to achieve eslicarbazepine exposures in pediatric patients aged 4 to 17 years similar to those observed in adults treated at effectives doses of APTIOM [see Dosage and Administration (2.2)]. The apparent half-life of eslicarbazepine in plasma was 10-16 hours in pediatric patients with partial-onset seizures. Steady-state plasma concentrations are attained after 4 to 5 days of once- daily dosing.
The pharmacokinetics of eslicarbazepine in pediatric patients are similar when used as monotherapy or as adjunctive therapy for the treatment of partial- onset seizures.
Gender
Studies in healthy subjects and patients showed that pharmacokinetics of eslicarbazepine was not affected by gender.
Race
No clinically significant effect of race (Caucasian N=849, Black N=53, Asian N=65, and Other N=51) on the pharmacokinetics of eslicarbazepine was noted in a population pharmacokinetic analysis of pooled data from the clinical studies.
Renal Impairment
APTIOM metabolites are eliminated from the systemic circulation primarily by renal excretion. The extent of systemic exposure of eslicarbazepine following an 800 mg single dose was increased by 62% in patients with mild renal impairment (CrCl 50-80 mL/min), by 2-fold in patients with moderate renal impairment (CrCl 30-49 mL/min) and by 2.5-fold in patients with severe renal impairment (CrCl <30 mL/min) in comparison to the healthy subjects (CrCl >80 mL/min). Dosage adjustment is recommended in patients with creatinine clearance below 50 mL/min [see Dosage and Administration (2.4) and Use in Specific Populations (8.6)].
In patients with end stage renal disease, repeated hemodialysis removed APTIOM metabolites from systemic circulation.
Hepatic Impairment
The pharmacokinetics and metabolism of APTIOM was evaluated in healthy subjects and patients with moderate liver impairment (7-9 points on the Child- Pugh assessment) after multiple oral doses (see Figure 1). Moderate hepatic impairment did not affect the pharmacokinetics of APTIOM. No dose adjustment is recommended in patients with mild to moderate liver impairment.
The pharmacokinetics of APTIOM has not been studied in patients with severe hepatic impairment.
Figure 1: Impact of Intrinsic Factors on AUC of Eslicarbazepine
Drug Interaction Studies
Potential for Other AEDs to Affect Eslicarbazepine
The potential impact of other AEDs on the systemic exposure (area under the curve, AUC) of eslicarbazepine, the active metabolite of APTIOM, is shown in Figure 2:
Figure 2: Potential Impact of Other AEDs on AUC of Eslicarbazepine
Potential for APTIOM to Affect Other Drugs
The potential impact of APTIOM on the systemic exposure (AUC) of other drugs (including AEDs) is shown in Figures 3a and 3b:
Figure 3a: Potential Impact of APTIOM on the AUC of AEDs
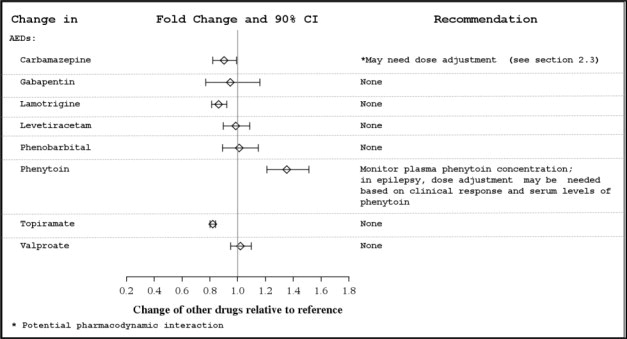
Figure 3b: Potential Impact of APTIOM on the AUC of Non-AEDs
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
In a two-year carcinogenicity study in mice, eslicarbazepine acetate was administered orally at doses of 100, 250, and 600 mg/kg/day. An increase in the incidence of hepatocellular adenomas and carcinomas was observed at 250 and 600 mg/kg/day in males and at 600 mg/kg/day in females. The dose not associated with an increase in tumors (100 mg/kg/day) is less than the MRHD (1600 mg/day for monotherapy) on a mg/m2 basis.
Mutagenesis
Eslicarbazepine acetate and eslicarbazepine were not mutagenic in the in vitro Ames assay. In in vitro assays in mammalian cells, eslicarbazepine acetate and eslicarbazepine were not clastogenic in human peripheral blood lymphocytes; however, eslicarbazepine acetate was clastogenic in Chinese hamster ovary (CHO) cells, with and without metabolic activation. Eslicarbazepine acetate was positive in the in vitro mouse lymphoma tk assay in the absence of metabolic activation. Eslicarbazepine acetate was not clastogenic in the in vivo mouse micronucleus assay.
Impairment of Fertility
When eslicarbazepine acetate (150, 350, and 650 mg/kg/day) was orally administered to male and female mice prior to and throughout the mating period, and continuing in females to gestation day 6, there was an increase in embryolethality at all doses. The lowest dose tested is less than the MRHD on a mg/m2 basis.
When eslicarbazepine acetate (65, 125, 250 mg/kg/day) was orally administered to male and female rats prior to and throughout the mating period, and continuing in females to implantation, lengthening of the estrus cycle was observed at the highest dose tested. The data in rats are of uncertain relevance to humans because of differences in metabolic profile between species.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
14.1 Monotherapy for Partial-Onset Seizures
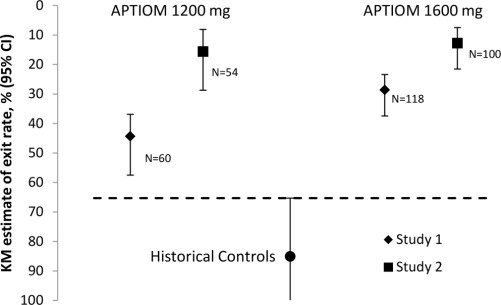
The effectiveness of APTIOM as monotherapy for partial-onset seizures was established in two identical, dose-blinded historical control trials in a total of 365 patients with epilepsy (Study 1 and Study 2). In these trials, patients were randomized in a 2:1 ratio to receive either APTIOM 1600 mg or 1200 mg once daily, and their responses were compared to those of a historical control group. The historical control methodology is described in a publication by French et al. [see References (15)]. The historical control consisted of a pooled analysis of the control groups from 8 trials of similar design, which utilized a subtherapeutic dose of an AED as a comparator. Statistical superiority to the historical control was considered to be demonstrated if the upper limit from a 2-sided 95% confidence interval for the percentage of patients meeting exit criteria in patients receiving APTIOM remained below the lower 95% prediction interval of 65% derived from the historical control data.
In Study 1 and Study 2, patients ≥16 years of age experienced at least 4 seizures during the baseline period with no 28-day seizure free period while receiving 1 or 2 AEDs (both could not be sodium-channel blocking drugs, and at least one AED was limited to 2/3 of a typical dose). APTIOM was titrated over a 1- to 2-week period followed by the gradual withdrawal of the background AED over a 6-week period, followed by a 10-week monotherapy period.
The exit criteria were one or more of the following: (1) an episode of status epilepticus, (2) emergence of a generalized tonic-clonic seizure in patients who had not had one in the past 6 months, (3) doubling of average monthly seizure count during any 28 consecutive days, (4) doubling of highest consecutive 2-day seizure frequency during the entire treatment phase, or (5) worsening of seizure severity considered by the investigator to require intervention. The primary endpoint was the cumulative 112-day exit rate in the efficacy population. Additionally, in Studies 1 and 2, if the discontinuation rate exceeded 10%, patients were randomly reassigned to be counted as exits.
The most commonly used baseline AEDs were carbamazepine, levetiracetam, valproic acid, and lamotrigine. Oxcarbazepine was used as a baseline AED in 6.6% of patients.
In Study 1, the Kaplan-Meier (K-M) estimate of the percentage of patients meeting at least 1 exit criterion was 29% (95% CI: 21%, 38%) in the 1600 mg group and 44% (95% CI 33%, 58%) in the 1200 mg group. In Study 2, the K-M estimate of the percentage of patients meeting at least 1 exit criterion was 13% (95% CI: 8%, 22%) in the 1600 mg group and 16% (95% CI: 8%, 29%) in the 1200 mg group. The upper limit of the 2-sided 95% CI of both doses in both trials were below the threshold of 65% derived from the historical control data, meeting the pre-specified criteria for efficacy (see Figure 4).
Figure 4: Kaplan-Meier Estimates of Cumulative 112-Day Exit Rates for Studies 1 and 2
14.2 Adjunctive Therapy for Partial-Onset Seizures
The efficacy of APTIOM as adjunctive therapy in partial-onset seizures was established in three randomized, double-blind, placebo-controlled, multicenter trials in adult patients with epilepsy (Study 3, Study 4, and Study 5). Patients enrolled had partial-onset seizures with or without secondary generalization and were not adequately controlled with 1 to 3 concomitant AEDs. During an 8-week baseline period, patients were required to have an average of ≥4 partial-onset seizures per 28 days with no seizure-free period exceeding 21 days. In these three trials, patients had a median duration of epilepsy of 19 years and a median baseline seizure frequency of 8 seizures per 28 days. Two-thirds (69%) of subjects used 2 concomitant AEDs and 28% used 1 concomitant AED. The most commonly used AEDs were carbamazepine (50%), lamotrigine (24%), valproic acid (21%), and levetiracetam (18%). Oxcarbazepine was not allowed as a concomitant AED.
Studies 3 and 4 compared dosages of APTIOM 400, 800, and 1200 mg once daily with placebo. Study 5 compared dosages of APTIOM 800 and 1200 mg once daily with placebo. In all three trials, following an 8-week Baseline Phase, which established a baseline seizure frequency, subjects were randomized to a treatment arm. Patients entered a treatment period consisting of an initial titration phase (2 weeks), and a subsequent maintenance phase (12 weeks). The specific titration schedule differed amongst the three studies. Thus, patients were started on a daily dose of 400 mg or 800 mg and subsequently increased by 400 mg/day following one or two weeks, until the final daily target dose was achieved.
The standardized seizure frequency during the Maintenance Phase over 28 days was the primary efficacy endpoint in all three trials. Table 5 presents the results for the primary endpoint, as well as the secondary endpoint of percent reduction from baseline in seizure frequency. The APTIOM treatment at 400 mg/day was studied in Studies 3 and 4 and did not show significant treatment effect. A statistically significant effect was observed with APTIOM treatment at doses of 800 mg/day in Studies 3 and 4, but not in Study 5, and at doses of 1200 mg/day in all 3 studies.
Table 5: Standardized Seizure Frequency During the Maintenance Phase Over 28 Days and Percent Reduction from Baseline in Seizure Frequency|
Placebo |
APTIOM | ||
|---|---|---|---|
|
800 mg |
1200 mg | ||
|
*statistically significant compared to placebo | |||
|
Study 3 | |||
|
N |
95 |
88 |
87 |
|
Seizure Frequency (LS Mean seizures per 28 days) |
6.6 |
5.0 (0.047*) |
4.3 (0.001*) |
|
Median Percent Reduction from Baseline in Seizure Frequency (%) |
-15 |
-36 |
-39 |
|
Study 4 | |||
|
N |
99 |
87 |
81 |
|
Seizure Frequency (LS Mean seizures per 28 days) |
8.6 |
6.2 (0.006*) |
6.6 (0.042*) |
|
Median Percent Reduction from Baseline in Seizure Frequency (%) |
-6 |
-33 |
-28 |
|
Study 5 | |||
|
N |
212 |
200 |
184 |
|
Seizure Frequency (LS Mean seizures per 28 days) |
7.9 |
6.5 (0.058) |
6.0 (0.004*) |
|
Median Percent Reduction from Baseline in Seizure Frequency (%) |
-22 |
-30 |
-36 |
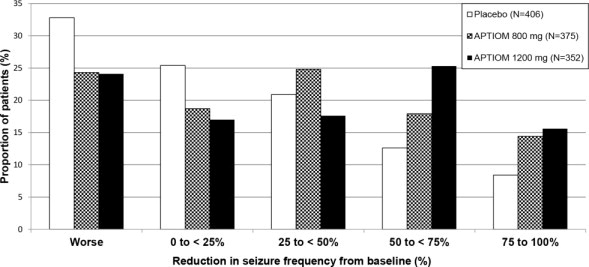
Figure 5 shows changes from baseline in the 28-day total partial seizure frequency by category of reduction in seizure frequency from baseline for patients treated with APTIOM and placebo in an integrated analysis across the three clinical trials. Patients in whom the seizure frequency increased are shown to the left as “Worse.” Patients in whom the seizure frequency decreased are shown in four categories.
Figure 5: Proportion of Patients by Category of Seizure Reduction for APTIOM and Placebo Across All Three Double-blind Trials
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
APTIOM tablets are white, oblong and with functional scoring on one side (200 mg, 600 mg, and 800 mg) or white, circular bi-convex and plain on one side (400 mg) and identified with strength-specific one-sided engraving on the other side, “ESL 200” (200 mg), “ESL 400” (400 mg), “ESL 600” (600 mg), or “ESL 800” (800 mg). Tablets are supplied in the following strengths and package configurations (Table 6):
Table 6: Package Configuration for APTIOM Tablets|
Tablet Strength |
Package Configuration |
NDC Code |
|
200 mg |
Bottles of 30 |
63402-202-30 |
|
400 mg |
Bottles of 30 |
63402-204-30 |
|
600 mg |
Bottles of 60 |
63402-206-60 |
|
800 mg |
Bottles of 30 |
63402-208-30 |
16.2 Storage and Handling
Store APTIOM tablets at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
Inform patients and caregivers of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking APTIOM. Instruct patients and caregivers that APTIOM should only be taken as prescribed.
Suicidal Behavior and Ideation
Counsel patients, their caregivers, and families that AEDs, including APTIOM, may increase the risk of suicidal thoughts and behavior and advise them of the need to be alert for the emergence or worsening of symptoms of depression, any unusual changes in mood or behavior, or the emergence of suicidal thoughts, behavior, or thoughts about self-harm. Instruct patients, caregivers, and families to report behaviors of concern immediately to healthcare providers [see Warnings and Precautions (5.1)].
Serious Dermatologic Reactions
Advise patients and caregivers about the risk of potentially fatal serious skin reactions. Educate patients and caregivers about the signs and symptoms that may signal a serious skin reaction. Instruct patients and caregivers to consult with their healthcare provider immediately if a skin reaction occurs during treatment with APTIOM [see Warnings and Precautions (5.2)].
DRESS/Multi-organ Hypersensitivity
Instruct patients and caregivers that a fever associated with signs of other organ system involvement (e.g., rash, lymphadenopathy, hepatic dysfunction) may be drug-related and should be reported to their healthcare provider immediately [see Warnings and Precautions (5.3)].
Anaphylactic Reactions and Angioedema
Advise patients and caregivers of life threatening symptoms suggesting anaphylaxis or angioedema (swelling of the face, eyes, lips, tongue, or difficulty in swallowing or breathing) that can occur with APTIOM. Instruct them to immediately report these symptoms to their healthcare provider [see Warnings and Precautions (5.4)].
Hyponatremia
Advise patients and caregivers that APTIOM may reduce serum sodium concentrations, especially if they are taking other medications that can lower sodium. Advise patients and caregivers to report symptoms of low sodium such as nausea, tiredness, lack of energy, irritability, confusion, muscle weakness/spasms, or more frequent or more severe seizures [see Warnings and Precautions (5.5)].
Neurological Adverse Reactions
Counsel patients and caregivers that APTIOM may cause dizziness, gait disturbance, somnolence/fatigue, cognitive dysfunction, and visual changes. These adverse reactions, if observed, are more likely to occur during the titration period compared to the maintenance period. Advise patients not to drive or operate machinery until they have gained sufficient experience on APTIOM to gauge whether it adversely affects their ability to drive or operate machinery [see Warnings and Precautions (5.6)].
Withdrawal of APTIOM
Advise patients and caregivers not to discontinue use of APTIOM without consulting with their healthcare provider. APTIOM should be gradually withdrawn to minimize the potential of increased seizure frequency and status epilepticus [see Warnings and Precautions (5.7)].
Hematologic Adverse Reactions
Advise patients and caregivers that there have been rare reports of blood disorders reported in patients treated with APTIOM. Instruct patients to immediately consult with their physician if they experience symptoms suggestive of blood disorders [see Warnings and Precautions (5.10)].
Interaction with Oral Contraceptives
Inform patients and caregivers that APTIOM can significantly decrease the effectiveness of hormonal contraceptives. Recommend that female patients of childbearing potential use additional or alternative non-hormonal forms of contraception during treatment with APTIOM and after treatment has been discontinued for at least one menstrual cycle or until otherwise instructed by their healthcare provider [see Drug Interactions (7.3, 7.4)].
Pregnancy Registry
Encourage patients to enroll in the North American Antiepileptic Drug Pregnancy Registry if they become pregnant. This Registry is collecting information about the safety of AEDs during pregnancy. To enroll, patients can call 1-888-233-2334 (toll-free) [see Use in Specific Populations (8.1)].

Manufactured for:
Sumitomo Pharma America, Inc.
Marlborough, MA 01752 USA
Under license from 
© 2023 Sumitomo Pharma America, Inc. All rights reserved.
10018-03
SPL MEDGUIDE SECTION
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. |
Revised 9/2017 | |||
|
MEDICATION GUIDE | ||||
|
What is the most important information I should know about APTIOM? *Do not stop taking APTIOM without first talking to your healthcare provider. * Stopping APTIOM suddenly can cause serious problems. Stopping a seizure medicine suddenly in a patient who has epilepsy may cause seizures that will not stop (status epilepticus). | ||||
|
1. Like other antiepileptic drugs, APTIOM may cause suicidal thoughts or
actions in a very small number of people, about 1 in 500. | ||||
|
| |||
|
How can I watch for early symptoms of suicidal thoughts and actions?
| ||||
|
2. APTIOM may cause allergic reactions or serious problems which may affect
organs and other parts of your body like the liver or blood cells. You may or
may not have a rash with these types of reactions. | ||||
|
| |||
|
*APTIOM may cause the level of sodium in your blood to be low. Symptoms of low blood sodium include: | ||||
|
| |||
|
Some medicines can also cause low sodium in your blood. Be sure to tell your healthcare provider about all the other medicines that you are taking. | ||||
|
What is APTIOM? | ||||
|
Who should not take APTIOM? | ||||
|
What should I tell my healthcare provider before taking APTIOM?
| ||||
|
Tell your healthcare provider about all the medicines you take, including
prescription and over-the-counter medicines, vitamins, and herbal supplements. | ||||
|
|
|
|
|
|
Ask your healthcare provider or pharmacist for a list of these medicines, if
you are not sure. | ||||
|
How should I take APTIOM?
| ||||
|
What should I avoid while taking APTIOM?
| ||||
|
What are the possible side effects of APTIOM? *Nervous system problems. APTIOM may cause problems that can affect your nervous system. Symptoms of nervous system problems include: | ||||
|
|
| ||
|
*Liver problems. APTIOM may affect your liver. Symptoms of liver problems include: | ||||
|
|
| ||
|
Get medical help right away if you have any of the symptoms listed above or
listed in “What is the most important information I should know about
APTIOM?” | ||||
|
|
|
|
|
|
Tell your healthcare provider if you have any side effect that bothers you or
that does not go away. | ||||
|
How should I store APTIOM?
| ||||
|
Keep APTIOM and all medicines out of reach of children. | ||||
|
What are the ingredients in APTIOM? | ||||
|
General information about the safe and effective use of APTIOM. | ||||
|
|
