Amlodipine and Benazepril Hydrochloride
These highlights do not include all the information needed to use AMLODIPINE AND BENAZEPRIL HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for AMLODIPINE AND BENAZEPRIL HYDROCHLORIDE CAPSULES. AMLODIPINE AND BENAZEPRIL HYDROCHLORIDE capsules, for oral use Initial U.S. Approval: 1995
1dd12925-ede8-4ad5-9309-d8c79da1cebf
HUMAN PRESCRIPTION DRUG LABEL
May 3, 2023
A-S Medication Solutions
DUNS: 830016429
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Amlodipine and Benazepril Hydrochloride
PRODUCT DETAILS
INGREDIENTS (13)
Drug Labeling Information
Warnings And Precautions Section
5 WARNINGS AND PRECAUTIONS
5.1 Fetal Toxicity
Amlodipine Benazepril hydrochloride can cause fetal harm when administered to a pregnant woman. Use of drugs that act on the renin-angiotensin system during the second and third trimesters of pregnancy reduces fetal renal function and increases fetal and neonatal morbidity and death. Resulting oligohydramnios can be associated with fetal lung hypoplasia and skeletal deformations. Potential neonatal adverse effects include skull hypoplasia, anuria, hypotension, renal failure, and death. When pregnancy is detected, discontinue amlodipine and benazepril hydrochloride as soon as possible [see Use in Specific Populations (8.1)].
5.2 Angioedema and Anaphylactoid Reactions
Head and Neck Angioedema: Angioedema of the face, extremities, lips, tongue, glottis, and larynx has been reported in patients treated with benazepril. This may occur at any time during treatment. Angioedema associated with edema of the larynx, tongue, or glottis can compromise the airway and be fatal. If laryngeal stridor or angioedema of the face, tongue, or glottis occurs, discontinue treatment with amlodipine and benazepril hydrochloride and treat immediately. When involvement of the tongue, glottis, or larynx appears likely to cause airway obstruction, appropriate therapy, e.g., administer subcutaneous epinephrine injection 1:1000 (0.3 to 0.5 mL), promptly [see Adverse Reactions (6)].
Patients with a history of angioedema may be at increased risk for angioedema while receiving amlodipine and benazepril hydrochloride. Black patients receiving ACE inhibitors have a higher incidence of angioedema compared to nonblacks.
Patients receiving coadministration of ACE inhibitor and mTOR (mammalian target of rapamycin) inhibitor (e.g., temsirolimus, sirolimus, everolimus) therapy or a neprilysin inhibitor may be at increased risk for angioedema [see Drug Interactions (7)].
Intestinal Angioedema: Intestinal angioedema has been reported in patients treated with ACE inhibitors. These patients presented with abdominal pain (with or without nausea or vomiting); in some cases there was no prior history of facial angioedema and C-1 esterase levels were normal. The angioedema was diagnosed by procedures including abdominal CT scan or ultrasound, or at surgery, and symptoms resolved after stopping the ACE inhibitor. Intestinal angioedema should be included in the differential diagnosis of patients on ACE inhibitors presenting with abdominal pain.
Anaphylactoid Reactions During Desensitization: Two patients undergoing desensitizing treatment with hymenoptera (wasp sting) venom while receiving ACE inhibitors sustained life-threatening anaphylactoid reactions.
Anaphylactoid Reactions During Membrane Exposure: Anaphylactoid reactions have been reported in patients dialyzed with high-flux membranes and treated concomitantly with an ACE inhibitor. Anaphylactoid reactions have also been reported in patients undergoing low-density lipoprotein apheresis with dextran sulfate absorption.
5.3 Increased Angina and/or Myocardial Infarction
Worsening angina and acute myocardial infarction can develop after starting or increasing the dose of amlodipine, particularly in patients with severe obstructive coronary artery disease.
5.4 Hypotension
Amlodipine and benazepril hydrochloride can cause symptomatic hypotension, sometimes complicated by oliguria, progressive azotemia, acute renal failure, or death. Symptomatic hypotension is most likely to occur in patients who have heart failure, severe aortic or mitral stenosis, obstructive hypertrophic cardiomyopathy or have been volume or salt depleted as a result of diuretic therapy, dietary salt restriction, dialysis, diarrhea, or vomiting. Correct volume and salt depletion before starting therapy with benazepril. If hypotension occurs, place the patient in the supine position and give physiological saline intravenously if needed. Continue treatment with benazepril once blood pressure and volume have returned to normal.
In patients with congestive heart failure, start amlodipine and benazepril hydrochloride therapy under close medical supervision; follow closely for the first 2 weeks of treatment and whenever the dose of the benazepril component is increased or a diuretic is added or its dose increased.
In patients undergoing surgery or during anesthesia with agents that produce hypotension, benazepril will block the angiotensin II formation that could otherwise occur secondary to compensatory renin release. Hypotension that occurs as a result of this mechanism can be corrected by volume expansion.
5.5 Impaired Renal Function
Monitor renal function periodically in patients treated with amlodipine and benazepril hydrochloride. Changes in renal function, including acute renal failure, can be caused by drugs that affect the RAS. Patients whose renal function may depend in part on the activity of the RAS (e.g., patients with renal artery stenosis, severe heart failure, post-myocardial infarction or volume depletion) or who are on Nonsteroidal Anti-Inflammatory Drugs (NSAIDs) or ARBs may be at particular risk of developing acute renal failure on amlodipine and benazepril hydrochloride. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on amlodipine and benazepril hydrochloride.
5.6 Hyperkalemia
Monitor serum potassium periodically in patients receiving amlodipine and benazepril hydrochloride. Drugs that affect the RAS can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, and/or potassium-containing salt substitutes. In U.S. placebo-controlled trials of amlodipine and benazepril hydrochloride, hyperkalemia [serum potassium at least 0.5 mEq/L greater than the upper limit of normal (ULN)] not present at baseline occurred in approximately 1.5% of hypertensive patients receiving amlodipine and benazepril hydrochloride. Increases in serum potassium were generally reversible.
5.7 Hepatitis and Hepatic Failure
There have been rare reports of predominantly cholestatic hepatitis and isolated cases of acute liver failure, some of them fatal, in patients on ACE inhibitors. The mechanism is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevation of hepatic enzymes should discontinue the ACE inhibitor and be kept under medical surveillance.
- Anaphylactoid reactions, including angioedema. (5.2)
- Myocardial infarction or increased angina in patients with obstructive coronary artery disease. (5.3)
- Assess for hypotension and hyperkalemia. (5.4, 5.6)
- Titrate slowly in patients with impaired hepatic or severely impaired renal function. (5.5, 5.7)
Adverse Reactions Section
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Amlodipine and benazepril hydrochloride has been evaluated for safety in over 2,991 patients with hypertension; over 500 of these patients were treated for at least 6 months, and over 400 were treated for more than 1 year.
In a pooled analysis of 5 placebo-controlled trials involving amlodipine and benazepril hydrochloride doses up to 5/20, the reported side effects were generally mild and transient, and there was no relationship between side effects and age, sex, race, or duration of therapy. Discontinuation of therapy due to side effects was required in approximately 4% of patients treated with amlodipine and benazepril hydrochloride and in 3% of patients treated with placebo.
The most common reasons for discontinuation of therapy with amlodipine and benazepril hydrochloride in these studies were cough and edema (including angioedema).
The peripheral edema associated with amlodipine use is dose-dependent. When benazepril is added to a regimen of amlodipine, the incidence of edema is substantially reduced.
The addition of benazepril to a regimen of amlodipine should not be expected to provide additional antihypertensive effect in African-Americans. However, all patient groups benefit from the reduction in amlodipine-induced edema.
The side effects considered possibly or probably related to study drug that occurred in these trials in more than 1% of patients treated with amlodipine and benazepril hydrochloride are shown in the table below. Cough was the only adverse event with at least possible relationship to treatment that was more common on amlodipine and benazepril hydrochloride (3.3%) than on placebo (0.2%).
Percent Incidence in U.S. Placebo-controlled Trials|
*Edema refers to all edema, such as dependent edema, angioedema, facial edema. | ||||
|
Benazepril and Amlodipine |
Benazepril |
Amlodipine |
Placebo | |
|
N = 760 |
N = 554 |
N = 475 |
N = 408 | |
|
Cough |
3.3 |
1.8 |
0.4 |
0.2 |
|
Headache |
2.2 |
3.8 |
2.9 |
5.6 |
|
Dizziness |
1.3 |
1.6 |
2.3 |
1.5 |
|
Edema* |
2.1 |
0.9 |
5.1 |
2.2 |
The incidence of edema was greater in patients treated with amlodipine monotherapy (5.1%) than in patients treated with amlodipine and benazepril hydrochloride (2.1%) or placebo (2.2%).
Other side effects considered possibly or probably related to study drug that occurred in U.S. placebo-controlled trials of patients treated with amlodipine and benazepril hydrochloride or in postmarketing experience were the following:
Body as a Whole: Asthenia and fatigue.
CNS: Insomnia, nervousness, anxiety, tremor, and decreased libido.
Dermatologic: Flushing, hot flashes, rash, skin nodule, and dermatitis.
Digestive: Dry mouth, nausea, abdominal pain, dyspepsia, and esophagitis.
Hematologic: Neutropenia.
Musculoskeletal: cramps, and muscle cramps.
Urogenital: Sexual problems, such as impotence, and polyuria.
Monotherapies of benazepril and amlodipine have been evaluated for safety in clinical trials in over 6,000 and 11,000 patients, respectively. The observed adverse reactions to the monotherapies in these trials were similar to those seen in trials of amlodipine and benazepril hydrochloride.
6.2 Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In postmarketing experience with benazepril, there have been rare reports of Stevens-Johnson syndrome, pancreatitis, hemolytic anemia, pemphigus, thrombocytopenia, paresthesia, dysgeusia, orthostatic symptoms and hypotension, angina pectoris and arrhythmia, pruritus, photosensitivity reaction, arthralgia, arthritis, myalgia, blood urea nitrogen (BUN) increase, serum creatinine increase, renal impairment, vision impairment, agranulocytosis, neutropenia.
Rare reports in association with use of amlodipine: gingival hyperplasia, tachycardia, jaundice, and hepatic enzyme elevations (mostly consistent with cholestasis severe enough to require hospitalization), leukocytopenia, allergic reaction, hyperglycemia, dysgeusia, hypoesthesia, paresthesia, syncope, peripheral neuropathy, hypertonia, visual impairment, diplopia, hypotension, vasculitis, rhinitis, gastritis, hyperhidrosis, pruritus, skin discoloration, urticaria, erythema multiform, muscle spasms, arthralgia, micturition disorder, nocturia, erectile dysfunction, malaise, weight decrease or gain.
Other potentially important adverse experiences attributed to other ACE inhibitors and calcium channel blockers include: eosinophilic pneumonitis (ACE inhibitors) and gynecomastia (CCBs).
Discontinuation because of adverse reactions occurred in 4% of amlodipine and benazepril hydrochloride-treated patients and 3% of placebo-treated patients. The most common reasons for discontinuation of therapy with amlodipine and benazepril hydrochloride were cough and edema. (6.1)
** To report SUSPECTED ADVERSE REACTIONS, contact Rising Health, LLC at 1-833-395-6928 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.**
Dosage & Administration Section
2 DOSAGE AND ADMINISTRATION
2.1 General Considerations
The recommended initial dose is amlodipine 2.5 mg and benazepril 10 mg orally once-daily.
Begin therapy with amlodipine and benazepril hydrochloride capsules only after a patient has either (a) failed to achieve the desired antihypertensive effect with amlodipine or benazepril monotherapy, or (b) demonstrated inability to achieve adequate antihypertensive effect with amlodipine therapy without developing edema.
The antihypertensive effect of amlodipine and benazepril hydrochloride capsules is largely attained within 2 weeks. If blood pressure remains uncontrolled, the dose may be titrated up to amlodipine 10 mg and benazepril 40 mg once-daily. The dosing should be individualized and adjusted according to the patient’s clinical response.
In clinical trials of amlodipine and benazepril combination therapy using amlodipine doses of 2.5 to 10 mg and benazepril doses of 10 to 40 mg, the antihypertensive effects increased with increasing dose of amlodipine in all patient groups, and the effects increased with increasing dose of benazepril in nonblack groups.
2.2 Replacement Therapy
Amlodipine and benazepril hydrochloride capsules may be substituted for the titrated components.
- Usual starting dose is 2.5 mg/10 mg. (2.1)
- May be used as add-on therapy for patients not adequately controlled with either a dihydropyridine calcium channel blocker or an ACE inhibitor (2.2)
- Patients who experience edema with amlodipine may be switched to amlodipine and benazepril hydrochloride capsules containing a lower dose of amlodipine. (2.1)
Description Section
11 DESCRIPTION
Amlodipine and benazepril hydrochloride capsules USP are a combination of amlodipine besylate and benazepril hydrochloride.
Benazepril hydrochloride USP is a white to off-white, crystalline powder, soluble (greater than 100 mg/mL) in water, in ethanol, and in methanol. Benazepril hydrochloride’s chemical name is 3-[[1-(ethoxycarbonyl)-3-phenyl-(1S)-propyl]amino]-2,3,4,5-tetrahydro-2-oxo-1H-1-(3S)-benzazepine-1-acetic acid monohydrochloride; its structural formula is:

Its molecular formula is C24H28N2O5•HCl, and its molecular weight is 460.96.
Benazeprilat, the active metabolite of benazepril, is a nonsulfhydryl ACE inhibitor. Benazepril is converted to benazeprilat by hepatic cleavage of the ester group.
Amlodipine besylate USP is a white or almost white powder, slightly soluble in water and sparingly soluble in ethanol. Its chemical name is (R,S)3-ethyl-5-methyl-2-(2-aminoethoxymethyl)-4-(2-chlorophenyl)-1,4-dihydro-6-methyl-3,5-pyridinedicarboxylate benzenesulfonate; its structural formula is:
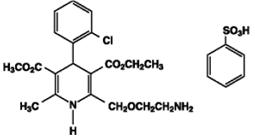
Its molecular formula is C20H25ClN2O5•C6H6O3S, and its molecular weight is 567.1.
Amlodipine besylate is the besylate salt of amlodipine, a dihydropyridine calcium channel blocker.
Amlodipine and benazepril hydrochloride is available as capsules containing amlodipine besylate USP (3.5 mg, 6.9 mg or 13.9 mg, equivalent to 2.5 mg, 5 mg or 10 mg of amlodipine respectively), with 10 mg, 20 mg, or 40 mg of benazepril hydrochloride USP providing for the following available combinations: 2.5 mg/10 mg, 5 mg/10 mg, 5 mg/20 mg, 5 mg/40 mg, 10 mg/20 mg, and 10 mg/40 mg.
The inactive ingredients of the capsules are colloidal silicon dioxide, crospovidone, gelatin, magnesium stearate, microcrystalline cellulose, povidone, sodium lauryl sulfate, and titanium dioxide. In addition, the hard gelatin capsule shells of 5 mg/10 mg contains iron oxide black, iron oxide red, and iron oxide yellow, 5 mg/20 mg contains iron oxide red, 5 mg/40 mg and 10 mg/40 mg contains FD&C Blue 1, FD&C Red 3, and 10 mg/20 mg contains D&C Red 28, FD&C Blue 1, FD&C Red 40, and FD&C Yellow 5. The capsules are printed with edible ink containing black iron oxide and shellac.
Nonclinical Toxicology Section
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with this combination. However, these studies have been conducted with amlodipine and benazepril alone (see below). No adverse effects on fertility occurred when the benazepril:amlodipine combination was given orally to rats of either sex at doses up to 15:7.5 mg (benazepril:amlodipine)/kg/day, prior to mating and throughout gestation.
Benazepril: No evidence of carcinogenicity was found when benazepril was administered to rats and mice for up to 2 years at doses of up to 150 mg/kg/day. When compared on the basis of body surface area, this dose is 18 and 9 times (rats and mice, respectively) the maximum recommended human dose (MRHD) (calculations assume a patient weight of 60 kg). No mutagenic activity was detected in the Ames test in bacteria, in an in vitro test for forward mutations in cultured mammalian cells, or in a nucleus anomaly test. At doses of 50 to 500 mg/kg/day (6 to 60 times the MRHD on a body surface area basis), benazepril had no adverse effect on the reproductive performance of male and female rats.
Amlodipine: Rats and mice treated with amlodipine maleate in the diet for up to 2 years, at concentrations calculated to provide daily dosage levels of 0.5, 1.25, and 2.5 mg amlodipine/kg/day, showed no evidence of a carcinogenic effect of the drug. For the mouse, the highest dose was, on a body surface area basis, similar to the MRHD of 10 mg amlodipine/day. For the rat, the highest dose was, on a body surface area basis, about two and a half times the MRHD (Calculations based on a 60 kg patient). Mutagenicity studies conducted with amlodipine maleate revealed no drug-related effects at either the gene or chromosome level. There was no effect on the fertility of rats treated orally with amlodipine maleate (males for 64 days and females for 14 days prior to mating) at doses of up to 10 mg amlodipine/kg/day (about 10 times the MRHD of 10 mg/day on a body surface area basis).
