Registrants1
Companies and organizations registered with the FDA for this drug approval, including their contact information and regulatory details.
Consumer Product Partners, LLC
119091520
Manufacturing Establishments1
FDA-registered manufacturing facilities and establishments involved in the production, packaging, or distribution of this drug product.
Consumer Product Partners, LLC
PUBLIX SUPER MARKETS, INC.
Consumer Product Partners, LLC
119091514
Products1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Magnesium Citrate
Product Details
Drug Labeling Information
Complete FDA-approved labeling information including indications, dosage, warnings, contraindications, and other essential prescribing details.
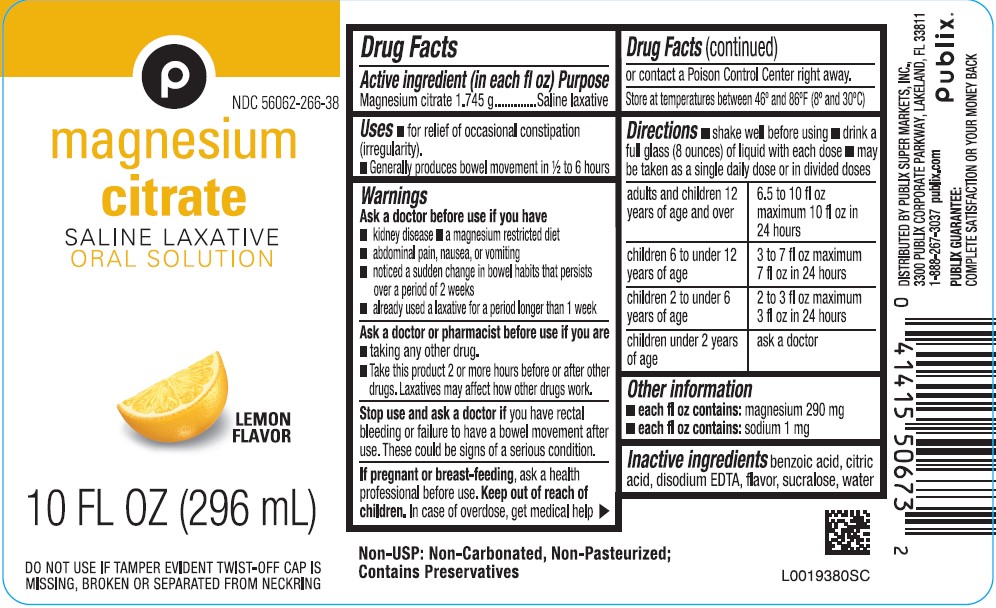
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
P
NDC 56062-266-38
magnesium citrate
SALINE LAZATIVE
ORAL SOLUTION
LEMON FLAVOR
10 FL OZ (296 mL)
DO NOT USE IF TAMPER EVIDENT TWIST-OF CAP IS MISSING, BROKEN OR SEPARATED FROM NECKRING
INDICATIONS & USAGE SECTION
Uses
- for relief of occasional constipation (irregularity)
- Generally produces bowel movement in 1/2 to 6 hours
DOSAGE & ADMINISTRATION SECTION
Directions
- shake well before using
- drink a full glass (8 ounces) of liquid with each dose
- may be taken as a single daily dose or in divide doses
adults and children 12 years of age and over - 6.5 to 10 fl oz maximum 10 fl oz in 24 hours
children 6 to under 12 years of age - 3 to 7 fl oz maximum 7 fl oz in 24 hours
children 2 to under 6 years of age - 2 to 3 fl oz maximum 3 fl oz in 24 hours
children under 2 years of age - ask a doctor
WARNINGS SECTION
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium restricted diet
- abdominal pain, nausea, or vomiting
- noticed a sudden change in bowel habits that persists over a period of 2 weeks
- already used a laxative for a period longer than 1 week
ADVERSE REACTIONS SECTION
ADVERSE REACTION
Non-USP: Non-Carbonated, Non-Pasteurized:
Contians Preservatives
DISTRIBUTED BY PUBLIX SUPER MARKETS, INC.,
3300 PUBLIX CORPORATE PARKWAY, LAKELAND, FL 33811
1-888-267-3037 publix.com
PUBLIX
PUBLIX GUARANTEE:
COMPLETE SATISFACTION OR YOUR MONEY BACK
STORAGE AND HANDLING SECTION
Storage
Store at temperature between 46° and 86°F (8° abd 30°C)
OTC - ACTIVE INGREDIENT SECTION
Active ingredient (in each fl oz)
Magnesium citrate 1.745 g
OTC - PURPOSE SECTION
Purpose
Saline Laxative
OTC - ASK DOCTOR/PHARMACIST SECTION
Ask a doctor or pharmacist before use if you are
- taking any other drug.
- Take this product 2 or more hours before or after other drugs. Laxatives may affect how other drugs work.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children
In case of overdose, get medical help or contact a Poison Control Center right away.
OTC - STOP USE SECTION
Stop use and ask a doctor
if you have rectal bleeding or failure to have a bowel movement after use. These could be signs of a serious condition.
OTC - PREGNANCY OR BREAST FEEDING SECTION
If pregnant or breast-feeding,
ask a health professional before use.
SPL UNCLASSIFIED SECTION
Other information
- each fl oz contains: magnesium 290 mg
- each fl oz contains: sodium 1 mg
INACTIVE INGREDIENT SECTION
Inactive ingredients
benzoic acid, citric acid, disodium EDTA, flavor, sucralose, water
