Nalbuphine Hydrochloride
a99fe500-f52b-483c-807c-178f1a78a02b
HUMAN PRESCRIPTION DRUG LABEL
Jan 18, 2024
Hospira, Inc.
DUNS: 141588017
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
NALBUPHINE HYDROCHLORIDE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
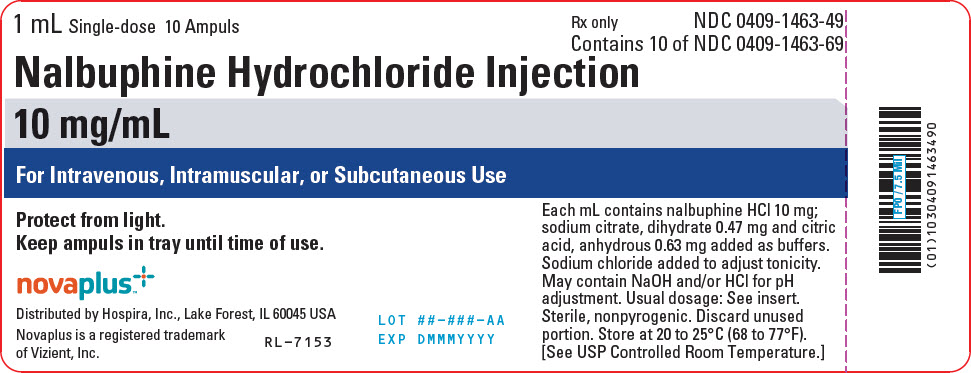
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 10 mg/mL Ampule Tray Label
1 mL Single-dose 10 Ampuls
Rx only
NDC 0409-1463-49
Contains 10 of NDC 0409-1463-69
Nalbuphine Hydrochloride Injection
10 mg/mL
For Intravenous, Intramuscular, or Subcutaneous Use
Protect from light.
Keep ampuls in tray until time of use.
Each mL contains nalbuphine HCl 10 mg;
sodium citrate, dihydrate 0.47 mg and citric
acid, anhydrous 0.63 mg added as buffers.
Sodium chloride added to adjust tonicity.
May contain NaOH and/or HCl for pH
adjustment. Usual dosage: See insert.
Sterile, nonpyrogenic. Discard unused
portion. Store at 20 to 25°C (68 to 77°F).
[See USP Controlled Room Temperature.]
novaplus™
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
Novaplus is a registered trademark
of Vizient, Inc.
RL-7153
LOT ##-###-AA
EXP DMMMYYYY
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
Important Dosage and Administration Instructions
Nalbuphine Hydrochloride Injection should be administered as a supplement to general anesthesia only by persons specifically trained in the use of intravenous anesthetics and management of the respiratory effects of potent opioids.
Naloxone, resuscitative and intubation equipment, and oxygen should be readily available.
Use the lowest effective dosage for the shortest duration of time consistent with individual patient treatment goals [seeWARNINGS]. Because the risk of overdose increases as opioid doses increase, reserve titration to higher doses of Nalbuphine Hydrochloride Injection for patients in whom lower doses are insufficiently effective and in whom the expected benefits of using a higher dose opioid clearly outweigh the substantial risks.
There is variability in the opioid analgesic dose and duration needed to adequately manage pain due both to the cause of pain and to individual patient factors. Initiate the dosing regimen for each patient individually, taking into account the patient's severity of pain, patient response, prior analgesic treatment experience, and risk factors for addiction, abuse, and misuse [seeWARNINGS].
Respiratory depression can occur at any time during opioid therapy, especially when initiating and following dosage increases with Nalbuphine Hydrochloride Injection. Consider this risk when selecting an initial dose and when making dose adjustments [seeWARNINGS].
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Initial Dosage
The usual recommended adult dose is 10 mg for a 70 kg individual administered subcutaneously, intramuscularly, or intravenously; this dose may be repeated every 3 to 6 hours as necessary. Use the lowest dose necessary to achieve adequate analgesia. Titrate the dose based upon the individual patient’s response to their initial dose of Nalbuphine Hydrochloride Injection. Dosage should be adjusted according to the severity of the pain, physical status of the patient, and other medications which the patient may be receiving [see WARNINGS; Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants]. In nontolerant individuals, the recommended single maximum dose is 20 mg with a maximum total daily dose of 160 mg.
The use of Nalbuphine Hydrochloride Injection as a supplement to balanced anesthesia requires larger doses than those recommended for analgesia. Induction doses of nalbuphine hydrochloride range from 0.3 mg/kg to 3 mg/kg intravenously to be administered over a 10 to 15 minute period with maintenance doses of 0.25 to 0.5 mg/kg in single intravenous administrations as required. The use of Nalbuphine Hydrochloride Injection may be followed by respiratory depression which can be reversed with the opioid antagonist naloxone hydrochloride.
Titration and Maintenance of Therapy
Individually titrate Nalbuphine Hydrochloride Injection to a dose that provides adequate analgesia and minimizes adverse reactions. Continually reevaluate patients receiving Nalbuphine Hydrochloride Injection to assess the maintenance of pain control, signs and symptoms of opioid withdrawal, and other adverse reactions, as well as to reassess for the development of addiction, abuse, or misuse [seeWARNINGS]. Frequent communication is important among the prescriber, other members of the healthcare team, the patient, and the caregiver/family during periods of changing analgesic requirements, including initial titration.
If the level of pain increases after dosage stabilization, attempt to identify the source of increased pain before increasing the nalbuphine hydrochloride dosage. If after increasing the dosage, unacceptable opioid-related adverse reactions are observed (including an increase in pain after dosage increase), consider reducing the dosage [seeWARNINGS]. Adjust the dosage to obtain an appropriate balance between management of pain and opioid- related adverse events.
Discontinuation of Nalbuphine Hydrochloride Injection
When a patient who has been taking Nalbuphine Hydrochloride Injection regularly and may be physically‑dependent no longer requires therapy with Nalbuphine Hydrochloride Injection, taper the dose gradually, by 25% to 50% every 2 to 4 days, while monitoring carefully for signs and symptoms of withdrawal. If the patient develops these signs or symptoms, raise the dose to the previous level and taper more slowly, either by increasing the interval between decreases, decreasing the amount of change in dose, or both. Do not abruptly discontinue Nalbuphine Hydrochloride Injection in a physically- dependent patient [see**WARNINGS****,**DRUG ABUSE AND DEPENDENCE].
HOW SUPPLIED SECTION
HOW SUPPLIED
Nalbuphine Hydrochloride Injection is supplied as a sterile solution in single-dose ampuls for intramuscular, subcutaneous, or intravenous administration, and available as follows:
|
Unit of Sale |
Concentration |
|---|---|
|
NDC 0409-1463-49 |
10 mg/mL |
Store at 20℃ to 25°C (68℉ to 77°F). [See USP Controlled Room Temperature.]
Protect from excessive light. Store in carton until contents have been used.
Distributed by Hospira, Inc., Lake Forest, IL 60045 USA
For Medical Information about Nalbuphine Hydrochloride Injection, please visit www.pfizermedinfo.com or call 1-800-438-1985.

Novaplus is a registered trademark of Vizient, Inc.
LAB-0838-12.0
Revised: 01/2024
