ACARBOSE
Acarbose Tablets 25 mg, 50 mg and 100 mg Rx Only
203cd13e-8c8d-4782-9e5a-2f9d1b20dd42
HUMAN PRESCRIPTION DRUG LABEL
May 1, 2022
Proficient Rx LP
DUNS: 079196022
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
ACARBOSE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
ACARBOSE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
ACARBOSE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 100 mg Tablet Bottle Label
NDC 71205-856-30
Acarbose
Tablets 100 mg
Rx only
30 Tablets

INDICATIONS & USAGE SECTION
INDICATIONS AND USAGE
Acarbose Tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
CONTRAINDICATIONS SECTION
CONTRAINDICATIONS
Acarbose Tablets are contraindicated in patients with known hypersensitivity to the drug. Acarbose Tablets are contraindicated in patients with diabetic ketoacidosis or cirrhosis. Acarbose Tablets are also contraindicated in patients with inflammatory bowel disease, colonic ulceration, partial intestinal obstruction or in patients predisposed to intestinal obstruction. In addition, Acarbose Tablets are contraindicated in patients who have chronic intestinal diseases associated with marked disorders of digestion or absorption and in patients who have conditions that may deteriorate as a result of increased gas formation in the intestine.
ADVERSE REACTIONS SECTION
ADVERSE REACTIONS
Digestive Tract:
Gastrointestinal symptoms are the most common reactions to Acarbose Tablets. In U.S. placebo-controlled trials, the incidences of abdominal pain, diarrhea, and flatulence were 19%, 31%, and 74% respectively in 1255 patients treated with Acarbose Tablets 50 to 300 mg t.i.d., whereas the corresponding incidences were 9%, 12%, and 29% in 999 placebo-treated patients.
In a one-year safety study, during which patients kept diaries of gastrointestinal symptoms, abdominal pain and diarrhea tended to return to pretreatment levels over time, and the frequency and intensity of flatulence tended to abate with time. The increased gastrointestinal tract symptoms in patients treated with Acarbose Tablets are a manifestation of the mechanism of action of Acarbose Tablets and are related to the presence of undigested carbohydrate in the lower GI tract.
If the prescribed diet is not observed, the intestinal side effects may be intensified. If strongly distressing symptoms develop in spite of adherence to the diabetic diet prescribed, the doctor must be consulted and the dose temporarily or permanently reduced.
Elevated Serum Transaminase Levels:
SeePRECAUTIONS.
Other Abnormal Laboratory Findings:
Small reductions in hematocrit occurred more often in Acarbose Tablets-treated patients than in placebo-treated patients but were not associated with reductions in hemoglobin. Low serum calcium and low plasma vitamin B6 levels were associated with Acarbose Tablets therapy but are thought to be either spurious or of no clinical significance.
Postmarketing Adverse Event Reports:
Additional adverse events reported from worldwide postmarketing experience include fulminant hepatitis with fatal outcome, hypersensitive skin reactions (e.g. rash, erythema, exanthema and urticaria), edema, ileus/subileus, jaundice and/or hepatitis and associated liver damage, thrombocytopenia, and pneumatosis cystoids intestinalis (seePRECAUTIONS.)
Pneumatosis Cystoides Intestinalis:
There have been rare postmarketing reports of pneumatosis cystoides intestinalis associated with the use of alpha-glucosidase inhibitors, including Acarbose Tablets. Pneumatosis cystoides intestinalis may present with symptoms of diarrhea, mucus discharge, rectal bleeding and constipation. Complications may include pneumoperitoneum, volvulus, intestinal obstruction, intussusception, intestinal hemorrhage and intestinal perforation. If pneumatosis cystoides intestinalis is suspected, discontinue Acarbose Tablets and perform the appropriate diagnostic imaging.
DESCRIPTION SECTION
DESCRIPTION
Acarbose Tablets are an oral alpha-glucosidase inhibitor for use in the management of type 2 diabetes mellitus. Acarbose is an oligosaccharide which is obtained from fermentation processes of a microorganism, Actinoplanes utahensis, and is chemically known as O-4,6-dideoxy-4-[[(1S,4R,5S,6S)-4,5,6-trihydroxy-3-(hydroxymethyl)-2-cyclohexen-1-yl]amino]-α-D- glucopyranosyl-(1→4)-O-α-D-glucopyranosyl-(1→4)-D-glucose. It is a white to off-white powder with a molecular weight of 645.6. Acarbose is soluble in water and has a pKa of 5.1. Its empirical formula is C25H43NO18 and its chemical structure is as follows:
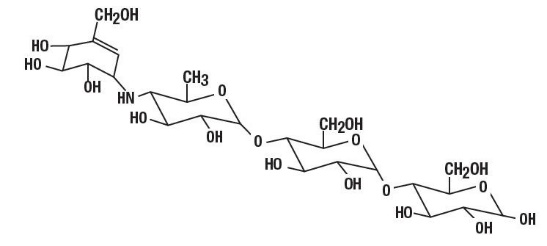
Acarbose Tablets are available as 25 mg, 50 mg and 100 mg tablets for oral use. The inactive ingredients are corn starch, magnesium stearate, microcrystalline cellulose and colloidal silicon dioxide.
CLINICAL PHARMACOLOGY SECTION
CLINICAL PHARMACOLOGY
Acarbose is a complex oligosaccharide that delays the digestion of ingested carbohydrates, thereby resulting in a smaller rise in blood glucose concentration following meals. As a consequence of plasma glucose reduction, Acarbose Tablets reduce levels of glycosylated hemoglobin in patients with type 2 diabetes mellitus. Systemic non-enzymatic protein glycosylation, as reflected by levels of glycosylated hemoglobin, is a function of average blood glucose concentration over time.
Mechanism of Action:
In contrast to sulfonylureas, Acarbose Tablets do not enhance insulin secretion. The antihyperglycemic action of acarbose results from a competitive, reversible inhibition of pancreatic alpha-amylase and membrane- bound intestinal alpha-glucoside hydrolase enzymes. Pancreatic alpha-amylase hydrolyzes complex starches to oligosaccharides in the lumen of the small intestine, while the membrane-bound intestinal alpha-glucosidases hydrolyze oligosaccharides, trisaccharides, and disaccharides to glucose and other monosaccharides in the brush border of the small intestine. In diabetic patients, this enzyme inhibition results in a delayed glucose absorption and a lowering of postprandial hyperglycemia.
Because its mechanism of action is different, the effect of Acarbose Tablets to enhance glycemic control is additive to that of sulfonylureas, insulin or metformin when used in combination. In addition, Acarbose Tablets diminish the insulinotropic and weight-increasing effects of sulfonylureas.
Acarbose has no inhibitory activity against lactase and consequently would not be expected to induce lactose intolerance.
Pharmacokinetics
Absorption:
In a study of 6 healthy men, less than 2% of an oral dose of acarbose was absorbed as active drug, while approximately 35% of total radioactivity from a 14C-labeled oral dose was absorbed. An average of 51% of an oral dose was excreted in the feces as unabsorbed drug-related radioactivity within 96 hours of ingestion. Because acarbose acts locally within the gastrointestinal tract, this low systemic bioavailability of parent compound is therapeutically desired. Following oral dosing of healthy volunteers with 14C-labeled acarbose, peak plasma concentrations of radioactivity were attained 14 to 24 hours after dosing, while peak plasma concentrations of active drug were attained at approximately 1 hour. The delayed absorption of acarbose-related radioactivity reflects the absorption of metabolites that may be formed by either intestinal bacteria or intestinal enzymatic hydrolysis.
Metabolism:
Acarbose is metabolized exclusively within the gastrointestinal tract, principally by intestinal bacteria, but also by digestive enzymes. A fraction of these metabolites (approximately 34% of the dose) was absorbed and subsequently excreted in the urine. At least 13 metabolites have been separated chromatographically from urine specimens. The major metabolites have been identified as 4 –methylpyrogallol derivatives (that is, sulfate, methyl, and glucuronide conjugates). One metabolite (formed by cleavage of a glucose molecule from acarbose) also has alpha-glucosidase inhibitory activity. This metabolite, together with the parent compound, recovered from the urine, accounts for less than 2% of the total administered dose.
Excretion:
The fraction of acarbose that is absorbed as intact drug is almost completely excreted by the kidneys. When acarbose was given intravenously, 89% of the dose was recovered in the urine as active drug within 48 hours. In contrast, less than 2% of an oral dose was recovered in the urine as active (this is, parent compound and active metabolite) drug. This is consistent with the low bioavailability of the parent drug. The plasma elimination half-life of acarbose activity is approximately 2 hours in healthy volunteers. Consequently, drug accumulation does not occur with three times a day (t.i.d.) oral dosing.
Special Populations:
The mean steady-state area under the curve (AUC) and maximum concentrations of acarbose were approximately 1.5 times higher in elderly compared to young volunteers; however, these differences were not statistically significant. Patients with severe renal impairment (Clcr < 25 mL/min/1.73m2) attained about 5 times higher peak plasma concentrations of acarbose and 6 times larger AUCs than volunteers with normal renal function. No studies of acarbose pharmacokinetic parameters according to race have been performed. In U.S. controlled clinical studies of Acarbose Tablets in patients with type 2 diabetes mellitus, reductions in glycosylated hemoglobin levels were similar in Caucasians (n=478) and African-Americans (n=167), with a trend toward a better response in Latinos (n=132).
Drug-Drug Interactions
Studies in healthy volunteers have shown that Acarbose Tablets have no effect on either the pharmacokinetics or pharmacodynamics of nifedipine, propranolol, or ranitidine. Acarbose Tablets did not interfere with the absorption or disposition of the sulfonylurea glyburide in diabetic patients. Acarbose Tablets may affect digoxin bioavailability and may require dose adjustment of digoxin by 16% (90% confidence interval: 8 to 23%), decrease mean Cmax of digoxin by 26% (90% confidence interval: 16 to 34%) and decreases mean trough concentrations of digoxin by 9% (90% confidence limit: 19% decrease to 2% increase). (SeePRECAUTIONS, Drug Interactions).
The amount of metformin absorbed while taking Acarbose Tablets was bioequivalent to the amount absorbed when taking placebo, as indicated by the plasma AUC values. However, the peak plasma level of metformin was reduced by approximately 20% when taking Acarbose Tablets due to a slight delay in the absorption of metformin. There is little if any clinically significant interaction between Acarbose Tablets and metformin.
CLINICAL STUDIES SECTION
CLINICAL TRIALS
Clinical Experience from Dose Finding Studies in Type 2 Diabetes Mellitus
Patients on Dietary Treatment Only
Results from six controlled, fixed-dose, monotherapy studies of Acarbose Tablets in the treatment of type 2 diabetes mellitus, involving 769 Acarbose Tablets-treated patients, were combined and a weighted average of the difference from placebo in the mean change from baseline in glycosylated hemoglobin (HbA1c) was calculated for each dose level as presented below:
Table 1
| |||
|
Mean Placebo-Subtracted Change in HbA1c in Fixed-Dose Monotherapy Studies | |||
|
Dose of Acarbose Tablets* |
N |
Change in HbA1c |
p-Value |
|
25 mg t.i.d. |
110 |
-0.44 |
0.0307 |
|
50 mg t.i.d. |
131 |
-0.77 |
0.0001 |
|
100 mg t.i.d. |
244 |
-0.74 |
0.0001 |
|
200 mg t.i.d.† |
231 |
-0.86 |
0.0001 |
|
300 mg t.i.d.† |
53 |
-1.00 |
0.0001 |
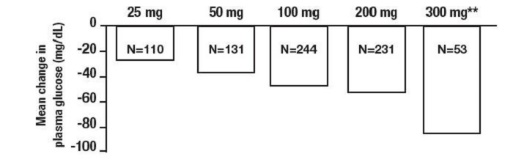
Results from these six fixed-dose, monotherapy studies were also combined to derive a weighted average of the difference from placebo in mean change from baseline for one-hour postprandial plasma glucose levels as shown in the following Figure.
|
|
** The 300 mg t.i.d. Acarbose Tablets regimen was superior to lower doses, but there were no statistically significant differences from 50 to 200 mg t.i.d. |
|
Figure 1 Dose of Acarbose Tablets (t.i.d.)* |
|
|
Clinical Experience in Type 2 Diabetes Mellitus Patients on Monotherapy, or
in Combination with Sulfonylureas, Metformin or Insulin
Acarbose Tablets were studied as monotherapy and as combination therapy to sulfonylurea, metformin, or insulin treatment. The treatment effects on HbA1c levels and one-hour postprandial glucose levels are summarized for four placebo-controlled, double-blind, randomized studies conducted in the United States in Tables 2 and 3, respectively. The placebo-subtracted treatment differences, which are summarized below, were statistically significant for both variables in all of these studies.
Study 1 (n=109) involved patients on background treatment with diet only. The mean effect of the addition of Acarbose Tablets to diet therapy was a change in HbA1c of -0.78%, and an improvement of one-hour postprandial glucose of -74.4 mg/dL.
In Study 2 (n=137), the mean effect of the addition of Acarbose Tablets to maximum sulfonylurea therapy was a change in HbA1c of -0.54%, and an improvement of one-hour postprandial glucose of -33.5 mg/dL.
In Study 3 (n=147), the mean effect of the addition of Acarbose Tablets to maximum metformin therapy was a change in HbA1c of -0.65%, and an improvement of one-hour postprandial glucose of -34.3 mg/dL.
Study 4 (n=145) demonstrated that Acarbose Tablets added to patients on background treatment with insulin resulted in a mean change in HbA1c of -0.69%, and an improvement of one-hour postprandial glucose of -36.0 mg/dL.
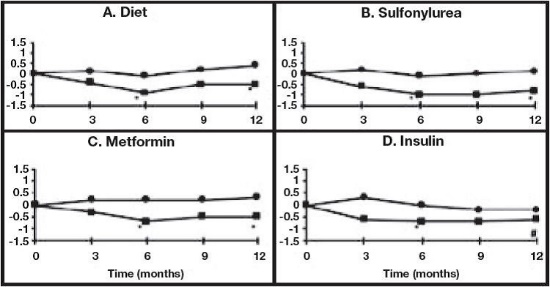
A one year study of Acarbose Tablets as monotherapy or in combination with sulfonylurea, metformin or insulin treatment was conducted in Canada in which 316 patients were included in the primary efficacy analysis (Figure 2). In the diet, sulfonylurea and metformin groups, the mean decrease in HbA1c produced by the addition of Acarbose Tablets was statistically significant at six months, and this effect was persistent at one year. In the Acarbose Tablets- treated patients on insulin, there was a statistically significant reduction in HbA1c at six months, and a trend for a reduction at one year.
Table 2: Effect of Acarbose Tablets on HbA1c|
HbA1c(%)* | |||||
|---|---|---|---|---|---|
|
Study |
Treatment |
Mean Baseline |
Mean change from baseline† |
Treatment Difference |
p-Value |
Þ | |||||
|
1 |
Placebo Plus Diet |
8.67 |
+0.33 |
|
|
|
Acarbose Tablets 100 mg t.i.d. Plus Diet |
8.69 |
-0.45 |
-0.78 |
0.0001 | |
|
2 |
Placebo Plus SFU‡ |
9.56 |
+0.24 |
|
|
|
Acarbose Tablets 50 to 300§ mg t.i.d. Plus SFU‡ |
9.64 |
-0.30 |
-0.54 |
0.0096 | |
|
3 |
Placebo Plus Metformin¶ |
8.17 |
+0.08# |
|
|
|
Acarbose Tablets 50 to 100 mg t.i.d Plus Metformin¶ |
8.46 |
-0.57# |
-0.65 |
0.0001 | |
|
4 |
Placebo Plus InsulinÞ |
8.69 |
+0.11 |
|
|
|
Acarbose Tablets 50 to 100 mg t.i.d. Plus InsulinÞ |
8.77 |
-0.58 |
-0.69 |
0.0001 |
| |||||
|
One-Hour Postprandial Glucose (mg/dL) | |||||
|
Study |
Treatment |
Mean Baseline |
Mean change from baseline* |
Treatment Difference |
p-Value |
|
1 |
Placebo Plus Diet |
297.1 |
+31.8 |
|
|
|
Acarbose Tablets 100 mg t.i.d. Plus Diet |
299.1 |
-42.6 |
-74.4 |
0.0001 | |
|
2 |
Placebo Plus SFU† |
308.6 |
+6.2 |
|
|
|
Acarbose Tablets 50 to 300‡ mg t.i.d. Plus SFU† |
311.1 |
-27.3 |
-33.5 |
0.0017 | |
|
3 |
Placebo Plus Metformin§ |
263.9 |
+3.3¶ |
|
|
|
Acarbose Tablets 50 to 100 mg t.i.d Plus Metformin§ |
283.0 |
-31.0¶ |
-34.3 |
0.0001 | |
|
4 |
Placebo Plus Insulin# |
279.2 |
+8.0 |
|
|
|
Acarbose Tablets 50 to 100 mg t.i.d. Plus Insulin# |
277.8 |
-28.0 |
-36.0 |
0.0178 |
|
Figure 2 |
|
|
Figure 2: Effects of Acarbose Tablets (▪) and Placebo (•) on mean change in HbA1c levels from baseline throughout a one-year study in patients with type 2 diabetes mellitus when used in combination with: (A) diet alone; (B) sulfonylurea; (C) metformin; or (D) insulin. Treatment differences at 6 and 12 months were tested:
- p < 0.01; # p = 0.077.
DOSAGE & ADMINISTRATION SECTION
DOSAGE AND ADMINISTRATION
There is no fixed dosage regimen for the management of diabetes mellitus with Acarbose Tablets or any other pharmacologic agent. Dosage of Acarbose Tablets must be individualized on the basis of both effectiveness and tolerance while not exceeding the maximum recommended dose of 100 mg t.i.d. Acarbose Tablets should be taken three times daily at the start (with the first bite) of each main meal. Acarbose Tablets should be started at a low dose, with gradual dose escalation as described below, both to reduce gastrointestinal side effects and to permit identification of the minimum dose required for adequate glycemic control of the patient. If the prescribed diet is not observed, the intestinal side effects may be intensified. If strongly distressing symptoms develop in spite of adherence to the diabetic diet prescribed, the doctor must be consulted and the dose temporarily or permanently reduced.
During treatment initiation and dose titration (see below), one-hour postprandial plasma glucose may be used to determine the therapeutic response to Acarbose Tablets and identify the minimum effective dose for the patient. Thereafter, glycosylated hemoglobin should be measured at intervals of approximately three months. The therapeutic goal should be to decrease both postprandial plasma glucose and glycosylated hemoglobin levels to normal or near normal by using the lowest effective dose of Acarbose Tablets, either as monotherapy or in combination with sulfonylureas, insulin or metformin.
Initial Dosage:
The recommended starting dosage of Acarbose Tablets is 25 mg given orally three times daily at the start (with the first bite) of each main meal. However, some patients may benefit from more gradual dose titration to minimize gastrointestinal side effects. This may be achieved by initiating treatment at 25 mg once per day and subsequently increasing the frequency of administration to achieve 25 mg t.i.d.
Maintenance Dosage:
Once a 25 mg t.i.d. dosage regimen is reached, dosage of Acarbose Tablets should be adjusted at 4 to 8 week intervals based on one-hour postprandial glucose or glycosylated hemoglobin levels, and on tolerance. The dosage can be increased from 25 mg t.i.d. to 50 mg t.i.d. Some patients may benefit from further increasing the dosage to 100 mg t.i.d. The maintenance dose ranges from 50 mg t.i.d. to 100 mg t.i.d. However, since patients with low body weight may be at increased risk for elevated serum transaminases, only patients with body weight > 60 kg should be considered for dose titration above 50 mg t.i.d. (seePRECAUTIONS). If no further reduction in postprandial glucose or glycosylated hemoglobin levels is observed with titration to 100 mg t.i.d., consideration should be given to lowering the dose. Once an effective and tolerated dosage is established, it should be maintained.
Maximum Dosage:
The maximum recommended dose for patient's ≤ 60 kg is 50 mg t.i.d. The maximum recommended dose for patients > 60 kg is 100 mg t.i.d.
Patients Receiving Sulfonylureas or Insulin:
Sulfonylurea agents or insulin may cause hypoglycemia. Acarbose Tablets given in combination with a sulfonylurea or insulin will cause a further lowering of blood glucose and may increase the potential for hypoglycemia. If hypoglycemia occurs, appropriate adjustments in the dosage of these agents should be made.
PRECAUTIONS SECTION
PRECAUTIONS
General
Macrovascular Outcomes:
There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with Acarbose Tablets or any other anti-diabetic drug.
Hypoglycemia:
Because of its mechanism of action, Acarbose Tablets when administered alone should not cause hypoglycemia in the fasted or postprandial state. Sulfonylurea agents or insulin may cause hypoglycemia. Because Acarbose Tablets given in combination with a sulfonylurea or insulin will cause a further lowering of blood glucose, it may increase the potential for hypoglycemia. Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, and no increased incidence of hypoglycemia was observed in patients when Acarbose Tablets were added to metformin therapy.
Oral glucose (dextrose), whose absorption is not inhibited by Acarbose Tablets, should be used instead of sucrose (cane sugar) in the treatment of mild to moderate hypoglycemia. Sucrose, whose hydrolysis to glucose and fructose is inhibited by Acarbose Tablets, is unsuitable for the rapid correction of hypoglycemia. Severe hypoglycemia may require the use of either intravenous glucose infusion or glucagon injection.
Elevated Serum Transaminase Levels:
In long-term studies (up to 12 months, and including Acarbose Tablets doses up to 300 mg t.i.d.) conducted in the United States, treatment-emergent elevations of serum transaminases (AST and/or ALT) above the upper limit of normal (ULN), greater than 1.8 times the ULN, and greater than 3 times the ULN occurred in 14%, 6%, and 3%, respectively, of Acarbose Tablets-treated patients as compared to 7%, 2%, and 1%, respectively, of placebo-treated patients. Although these differences between treatments were statistically significant, these elevations were asymptomatic, reversible, more common in females, and, in general, were not associated with other evidence of liver dysfunction. In addition, these serum transaminase elevations appeared to be dose related. In US studies including Acarbose Tablets doses up to the maximum approved dose of 100 mg t.i.d., treatment-emergent elevations of AST and/or ALT at any level of severity were similar between Acarbose Tablets-treated patients and placebo-treated patients (p ≥ 0.496).
In approximately 3 million patient-years of international postmarketing experience with Acarbose Tablets, 62 cases of serum transaminase elevations > 500 IU/L (29 of which were associated with jaundice) have been reported. Forty-one of these 62 patients received treatment with 100 mg t.i.d. or greater and 33 of 45 patients for whom weight was reported weighed < 60 kg. In the 59 cases where follow-up was recorded, hepatic abnormalities improved or resolved upon discontinuation of Acarbose Tablets in 55 and were unchanged in two. Cases of fulminant hepatitis with fatal outcome have been reported; the relationship to acarbose is unclear.
Loss of Control of Blood Glucose:
When diabetic patients are exposed to stress such as fever, trauma, infection, or surgery, a temporary loss of control of blood glucose may occur. At such times, temporary insulin therapy may be necessary.
Information for Patients:
Patients should be told to take Acarbose Tablets orally three times a day at the start (with the first bite) of each main meal. It is important that patients continue to adhere to dietary instructions, a regular exercise program, and regular testing of urine and/or blood glucose.
Acarbose Tablets itself does not cause hypoglycemia even when administered to patients in the fasted state. Sulfonylurea drugs and insulin, however, can lower blood sugar levels enough to cause symptoms or sometimes life- threatening hypoglycemia. Because Acarbose Tablets given in combination with a sulfonylurea or insulin will cause a further lowering of blood sugar, it may increase the hypoglycemic potential of these agents. Hypoglycemia does not occur in patients receiving metformin alone under usual circumstances of use, and no increased incidence of hypoglycemia was observed in patients when Acarbose Tablets were added to metformin therapy. The risk of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be well understood by patients and responsible family members. Because Acarbose Tablets prevent the breakdown of table sugar, patients should have a readily available source of glucose (dextrose, D-glucose) to treat symptoms of low blood sugar when taking Acarbose Tablets in combination with a sulfonylurea or insulin.
If side effects occur with Acarbose Tablets, they usually develop during the first few weeks of therapy. They are most commonly mild-to-moderate gastrointestinal effects, such as flatulence, diarrhea, or abdominal discomfort, and generally diminish in frequency and intensity with time.
Laboratory Tests:
Therapeutic response to Acarbose Tablets should be monitored by periodic blood glucose tests. Measurement of glycosylated hemoglobin levels is recommended for the monitoring of long-term glycemic control.
Acarbose Tablets, particularly at doses in excess of 50 mg t.i.d., may give rise to elevations of serum transaminases and, in rare instances, hyperbilirubinemia. It is recommended that serum transaminase levels be checked every 3 months during the first year of treatment with Acarbose Tablets and periodically thereafter. If elevated transaminases are observed, a reduction in dosage or withdrawal of therapy may be indicated, particularly if the elevations persist.
Monitoring glycemic control with 1,5-AG assay is not recommended as measurements of 1,5-AG are unreliable in assessing glycemic control in patients taking acarbose. Use alternative methods to monitor for glycemic control.
Renal Impairment:
Plasma concentrations of Acarbose Tablets in renally impaired volunteers were proportionally increased relative to the degree of renal dysfunction. Long- term clinical trials in diabetic patients with significant renal dysfunction (serum creatinine > 2 mg/dL) have not been conducted. Therefore, treatment of these patients with Acarbose Tablets is not recommended.
Drug Interactions
Certain drugs tend to produce hyperglycemia and may lead to loss of blood glucose control. These drugs include the thiazides and other diuretics, corticosteroids, phenothiazines, thyroid products, estrogens, oral contraceptives, phenytoin, nicotinic acid, sympathomimetics, calcium channel- blocking drugs, and isoniazid. When such drugs are administered to a patient receiving Acarbose Tablets, the patient should be closely observed for loss of blood glucose control. When such drugs are withdrawn from patients receiving Acarbose Tablets in combination with sulfonylureas or insulin, patients should be observed closely for any evidence of hypoglycemia.
Patients Receiving Sulfonylureas or Insulin:
Sulfonylurea agents or insulin may cause hypoglycemia. Acarbose Tablets given in combination with a sulfonylurea or insulin may cause a further lowering of blood glucose and may increase the potential for hypoglycemia. If hypoglycemia occurs, appropriate adjustments in the dosage of these agents should be made. Very rarely, individual cases of hypoglycemic shock have been reported in patients receiving Acarbose Tablets therapy in combination with sulfonylureas and/or insulin.
Intestinal adsorbents (e.g., charcoal) and digestive enzyme preparations containing carbohydrate-splitting enzymes (e.g., amylase, pancreatin) may reduce the effect of Acarbose Tablets and should not be taken concomitantly.
Acarbose Tablets have been shown to change the bioavailability of digoxin when they are coadministered, which may require digoxin dose adjustment. (See CLINICAL PHARMACOLOGY, Drug-Drug Interactions).
Carcinogenesis, Mutagenesis, and Impairment of Fertility.
Eight carcinogenicity studies were conducted with acarbose. Six studies were performed in rats (two strains, Sprague-Dawley and Wistar) and two studies were performed in hamsters.
In the first rat study, Sprague-Dawley rats received acarbose in feed at high doses (up to approximately 500 mg/kg body weight) for 104 weeks. Acarbose treatment resulted in a significant increase in the incidence of renal tumors (adenomas and adenocarcinomas) and benign Leydig cell tumors. This study was repeated with a similar outcome. Further studies were performed to separate direct carcinogenic effects of acarbose from indirect effects resulting from the carbohydrate malnutrition induced by the large doses of acarbose employed in the studies.
In one study using Sprague-Dawley rats, acarbose was mixed with feed but carbohydrate deprivation was prevented by the addition of glucose to the diet. In a 26-month study of Sprague-Dawley rats, acarbose was administered by daily postprandial gavage so as to avoid the pharmacologic effects of the drug. In both of these studies, the increased incidence of renal tumors found in the original studies did not occur. Acarbose was also given in food and by postprandial gavage in two separate studies in Wistar rats. No increased incidence of renal tumors was found in either of these Wistar rat studies. In two feeding studies of hamsters, with and without glucose supplementation, there was also no evidence of carcinogenicity.
Acarbose did not induce any DNA damage in vitro in the CHO chromosomal aberration assay, bacterial mutagenesis (Ames) assay, or a DNA binding assay. In vivo, no DNA damage was detected in the dominant lethal test in male mice, or the mouse micronucleus test.
Fertility studies conducted in rats after oral administration produced no untoward effect on fertility or on the overall capability to reproduce.
Pregnancy
Teratogenic Effects: Pregnancy Category B.
The safety of Acarbose Tablets in pregnant women has not been established. Reproduction studies have been performed in rats at doses up to 480 mg/kg (corresponding to 9 times the exposure in humans, based on drug blood levels) and have revealed no evidence of impaired fertility or harm to the fetus due to acarbose. In rabbits, reduced maternal body weight gain, probably the result of the pharmacodynamic activity of high doses of acarbose in the intestines, may have been responsible for a slight increase in the number of embryonic losses. However, rabbits given 160 mg/kg acarbose (corresponding to 10 times the dose in man, based on body surface area) showed no evidence of embryotoxicity and there was no evidence of teratogenicity at a dose 32 times the dose in man (based on body surface area). There are, however, no adequate and well-controlled studies of Acarbose Tablets in pregnant women. Because animal reproduction studies are not always predictive of the human response, this drug should be used during pregnancy only if clearly needed. Because current information strongly suggests that abnormal blood glucose levels during pregnancy are associated with a higher incidence of congenital anomalies as well as increased neonatal morbidity and mortality, most experts recommend that insulin be used during pregnancy to maintain blood glucose levels as close to normal as possible.
Nursing Mothers:
A small amount of radioactivity has been found in the milk of lactating rats after administration of radiolabeled acarbose. It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, Acarbose Tablets should not be administered to a nursing woman.
Pediatric Use:
Safety and effectiveness of Acarbose Tablets in pediatric patients have not been established.
Geriatric Use:
Of the total number of subjects in clinical studies of Acarbose Tablets in the United States, 27% were 65 and over, while 4% were 75 and over. No overall differences in safety and effectiveness were observed between these subjects and younger subjects. The mean steady-state area under the curve (AUC) and maximum concentrations of acarbose were approximately 1.5 times higher in elderly compared to young volunteers; however, these differences were not statistically significant.
OVERDOSAGE SECTION
OVERDOSAGE
Unlike sulfonylureas or insulin, an overdose of Acarbose Tablets will not result in hypoglycemia. An overdose may result in transient increases in flatulence, diarrhea, and abdominal discomfort which shortly subside. In cases of overdosage the patient should not be given drinks or meals containing carbohydrates (polysaccharides, oligosaccharides and disaccharides) for the next 4 to 6 hours.
HOW SUPPLIED SECTION
HOW SUPPLIED
Acarbose Tablets 25 mg are white to yellowish, round biconvex tablets marked "318" on one face and "cor" on the other face. They are supplied as follows:
|
Bottles of 30 |
NDC 71205-854-30 | |
|
Bottles of 60 |
NDC 71205-854-60 | |
|
Bottles of 90 |
NDC 71205-854-90 | |
|
Bottles of 100 |
NDC 71205-854-00 | |
|
Bottles of 120 |
NDC 71205-854-72 | |
|
Bottles of 180 |
NDC 71205-854-78 | |
|
Bottles of 500 |
NDC 71205-854-55 | |
|
Bottles of 1000 |
NDC 71205-854-11 |
Acarbose Tablets 50 mg are white to yellowish, round biconvex tablets marked "319" on one face and "cor" on the other face. They are supplied as follows:
|
Bottles of 30 |
NDC 71205-855-30 | |
|
Bottles of 60 |
NDC 71205-855-60 | |
|
Bottles of 90 |
NDC 71205-855-90 | |
|
Bottles of 100 |
NDC 71205-855-00 | |
|
Bottles of 120 |
NDC 71205-855-72 | |
|
Bottles of 180 |
NDC 71205-855-78 | |
|
Bottles of 500 |
NDC 71205-855-55 | |
|
Bottles of 1000 |
NDC 71205-855-11 |
Acarbose Tablets 100 mg are white to yellowish, round biconvex tablets marked "320" on one face and "cor" on the other face. They are supplied as follows:
|
Bottles of 30 |
NDC 71205-856-30 | |
|
Bottles of 60 |
NDC 71205-856-60 | |
|
Bottles of 90 |
NDC 71205-856-90 | |
|
Bottles of 100 |
NDC 71205-856-00 | |
|
Bottles of 120 |
NDC 71205-856-72 | |
|
Bottles of 180 |
NDC 71205-856-78 | |
|
Bottles of 500 |
NDC 71205-856-55 | |
|
Bottles of 1000 |
NDC 71205-856-11 |
Store at 20° to 25**°C (68° to 77°**F) [See USP Controlled Room Temperature].
Protect from moisture. Keep container tightly closed.
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
Rev: May 2018
Distributed By:
Virtus Pharmaceuticals, LLC
Langhorne, PA 19047
1-888-848-3593
Manufactured By:
Bluepharma Indústria Farmacêutica, S.A.
S. Martinho do Bispo
3045-016 Coimbra, Portugal
Repackaged and Relabeled By:
Proficient Rx LP
Thousand Oaks, CA 91320