Alfuzosin hydrochloride
These highlights do not include all the information needed to use ALFUZOSIN HYDROCHLORIDE EXTENDED-RELEASE TABLET safely and effectively. See full prescribing information for ALFUZOSIN HYDROCHLORIDE EXTENDED-RELEASE TABLETS. ALFUZOSIN hydrochloride extended-release tablets, for oral use Initial U.S. Approval: 2003
ffbd22eb-9df5-e0ad-e053-6294a90a7d4e
HUMAN PRESCRIPTION DRUG LABEL
Jul 5, 2023
Northwind Pharmaceuticals, LLC
DUNS: 036986393
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Alfuzosin hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
INDICATIONS & USAGE SECTION
1 INDICATIONS AND USAGE
Alfuzosin hydrochloride extended-release tablets, USP are indicated for the treatment of signs and symptoms of benign prostatic hyperplasia.
1.1 Important Limitations of Use
Alfuzosin hydrochloride extended-release tablets, USP are not indicated for the treatment of hypertension.
Alfuzosin hydrochloride extended-release tablets, USP are not indicated for use in pediatric population.
Alfuzosin hydrochloride extended-release tablets, USP, an alpha adrenergic antagonist, are indicated for the treatment of signs and symptoms of benign prostatic hyperplasia. ( 1)
Important Limitations of Use:
Alfuzosin hydrochloride extended-release tablets, USP are not indicated for treatment of hypertension. ( 1.1)
Alfuzosin hydrochloride extended-release tablets, USP are not indicated for use in pediatric population. ( 1.1, 8.4, 12.3)
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Postural Hypotension
Postural hypotension with or without symptoms (e.g., dizziness) may develop within a few hours following administration of alfuzosin hydrochloride extended-release tablets. As with other alpha adrenergic antagonists, there is a potential for syncope. Patients should be warned of the possible occurrence of such events and should avoid situations where injury could result should syncope occur. There may be an increased risk of hypotension/postural hypotension and syncope when taking alfuzosin hydrochloride extended-release tablets concomitantly with anti-hypertensive medication and nitrates. Care should be taken when alfuzosin hydrochloride extended-release tablets are administered to patients with symptomatic hypotension or patients who have had a hypotensive response to other medications.
5.2 Patients with Renal Impairment
Caution should be exercised when alfuzosin hydrochloride extended-release tablets are administered in patients with severe renal impairment (creatinine clearance < 30 mL/min) [see Use in Specific Populations (8.6)and Clinical Pharmacology (12.3)] .
5.3 Patients with Hepatic Impairment
Alfuzosin hydrochloride extended-release tablets are contraindicated for use in patients with moderate or severe hepatic impairment [see Contraindications (4), Use in Specific Populations (8.7)and Clinical Pharmacology (12.3)]. Although the pharmacokinetics of alfuzosin hydrochloride extended-release tablets have not been studied in patients with mild hepatic impairment, caution should be exercised when alfuzosin hydrochloride extended-release tablets are administered to such patients [see Use in Specific Populations (8.7)and Clinical Pharmacology (12.3)] .
5.4 Drug-Drug Interactions
Potent CYP3A4 Inhibitors: Alfuzosin hydrochloride extended-release tablets are contraindicated for use with potent CYP3A4 inhibitors (e.g. ketoconazole, itraconazole, ritonavir) since alfuzosin blood levels are increased [see Contraindications (4), Drug Interactions (7.1)and Clinical Pharmacology (12.3)].
**Other alpha adrenergic antagonists:**Alfuzosin hydrochloride extended- release tablets are an alpha adrenergic antagonist and should not be used in combination with other alpha adrenergic antagonist [see Drug Interactions (7.2)].
**Phosphodiesterase-5 (PDE5) Inhibitors:**PDE5-inhibitors are also vasodilators. Caution is advised for concomitant use of PDE5-inhibitors and alfuzosin hydrochloride extended-release tablets, as this combination can potentially cause symptomatic hypotension [see Drug Interactions (7.4)].
5.5 Prostatic Carcinoma
Carcinoma of the prostate and benign prostatic hyperplasia (BPH) cause many of the same symptoms. These two diseases frequently coexist. Therefore, patients thought to have BPH should be examined to rule out the presence of carcinoma of the prostate prior to starting treatment with alfuzosin hydrochloride extended-release tablets.
5.6 Intraoperative Floppy Iris Syndrome (IFIS)
IFIS has been observed during cataract surgery in some patients on or previously treated with alpha adrenergic antagonists. This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient's ophthalmologist should be prepared for possible modifications to their surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances.
There does not appear to be a benefit of stopping alpha adrenergic antagonist therapy prior to cataract surgery.
5.7 Priapism
Rarely (probably less than 1 in 50,000), alfuzosin, like other alpha adrenergic antagonists, has been associated with priapism (persistent painful penile erection unrelated to sexual activity). Because this condition can lead to permanent impotence if not properly treated, patients should be advised about the seriousness of the condition [see Adverse Reactions (6.2)and Patient Counseling Information (17.3)] .
5.8 Coronary Insufficiency
If symptoms of angina pectoris should appear or worsen, alfuzosin hydrochloride extended-release tablets should be discontinued.
5.9 Patients with Congenital or Acquired QT Prolongation
Use with caution in patients with acquired or congenital QT prolongation or who are taking medications that prolong the QT interval [see Clinical Pharmacology (12.2)].
- Postural hypotension/syncope: Care should be taken in patients with symptomatic hypotension or who have had a hypotensive response to other medications or are concomitantly treated with antihypertensive medication or nitrates ( 5.1)
- Use with caution in patients with severe renal impairment (creatinine clearance <30 mL/min) ( 5.2, 8.6, 12.3)
- Use with caution in patients with mild hepatic impairment ( 5.3, 8.7, 12.3)
- Should not be used in combination with other alpha adrenergic antagonists ( 5.4, 7.2)
- Prostate carcinoma should be ruled out prior to treatment ( 5.5)
- Intraoperative Floppy Iris Syndrome (IFIS) during cataract surgery may require modifications to the surgical technique ( 5.6)
- Discontinue alfuzosin hydrochloride extended-release tablets if symptoms of angina pectoris appear or worsen ( 5.8)
- Use with caution in patients with a history of QT prolongation or who are taking medications which prolong the QT interval ( 5.9, 12.2)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 CYP3A4 Inhibitors
Alfuzosin hydrochloride extended-release tablets are contraindicated for use with potent CYP3A4 inhibitors such as ketoconazole, itraconazole, or ritonavir, since alfuzosin blood levels are increased [see Contraindications (4), Warnings and Precautions (5.4)and Clinical Pharmacology (12.3)] .
7.2 Alpha Adrenergic Antagonists
The pharmacokinetic and pharmacodynamic interactions between alfuzosin hydrochloride extended-release tablets and other alpha adrenergic antagonists have not been determined. However, interactions may be expected, and alfuzosin hydrochloride extended-release tablets should not be used in combination with other alpha adrenergic antagonists [see Warnings and Precautions (5.4)].
7.3 Antihypertensive Medication and Nitrates
There may be an increased risk of hypotension/postural hypotension and syncope when taking alfuzosin hydrochloride extended-release tablets concomitantly with anti-hypertensive medication and nitrates [see Warnings and Precautions (5.1)] .
7.4 PDE5 Inhibitors
Caution is advised when alpha adrenergic antagonists, including alfuzosin hydrochloride extended-release tablets, are co-administered with PDE5 inhibitors. Alpha adrenergic antagonists and PDE5 inhibitors are both vasodilators that can lower blood pressure. Concomitant use of these two drug classes can potentially cause symptomatic hypotension [see Warnings and Precautions (5.4)] .
- Concomitant use of PDE5 inhibitors with alpha adrenergic antagonists, including alfuzosin hydrochloride extended-release tablets, can potentially cause symptomatic hypotension ( 5.4, 7.4)
DESCRIPTION SECTION
11 DESCRIPTION
Each alfuzosin hydrochloride extended-release tablet, USP contains 10 mg alfuzosin hydrochloride, USP as the active ingredient. Alfuzosin hydrochloride is a white to almost white powder that melts at approximately 240°C. It is freely soluble in water, sparingly soluble in alcohol, and practically insoluble in dichloromethane.
Alfuzosin hydrochloride is (R,S)-N-[3-[(4-amino-6,7-dimethoxy-2-quinazolinyl) methylamino]propyl]tetrahydro-2-furancarboxamide hydrochloride. The molecular formula of alfuzosin hydrochloride is C 19H 27N 5O 4•HCl. The molecular weight of alfuzosin hydrochloride is 425.9. Its structural formula is:
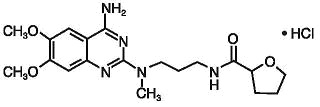
The tablet also contains the following inactive ingredients: hypromellose, lactose monohydrate, povidone, colloidal silicon dioxide and magnesium stearate.
Meets USP Dissolution test 4.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of a drug-related increase in the incidence of tumors in mice following dietary administration of 100 mg/kg/day alfuzosin for 98 weeks (13 and 15 times the maximum recommended human dose [MRHD] of 10 mg based on AUC of unbound drug), in females and males, respectively. The highest dose tested in female mice may not have constituted a maximally tolerated dose. Likewise, there was no evidence of a drug-related increase in the incidence of tumors in rats following dietary administration of 100 mg/kg/day alfuzosin for 104 weeks (53 and 37 times the MRHD in females and males, respectively).
Alfuzosin showed no evidence of mutagenic effect in the Ames and mouse lymphoma assays, and was free of any clastogenic effects in the Chinese hamster ovary cell and in vivomouse micronucleus assays. Alfuzosin treatment did not induce DNA repair in a human cell line.
There was no evidence of reproductive organ toxicity when male rats were administered oral doses of several hundred times (250 mg/kg/day for 26 weeks) the MRHD of alfuzosin. No impairment of fertility was observed following oral (gavage) administration to male rats at doses of up to 125 mg/kg/day for 70 days. Estrous cycling was inhibited in rats and dogs at approximately 12 and 18 times the MRHD respectively (doses of 25 mg/kg and 20 mg/kg, respectively), but did not result in impaired fertility in female rats.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Three randomized placebo-controlled, double-blind, parallel-arm, 12-week trials were conducted with the 10 mg daily dose of alfuzosin. In these three trials, 1,608 patients [mean age 64.2 years, range 49-92 years; Caucasian (96.1%), Black (1.6%), Asian (1.1%), Other (1.2%)] were randomized and 473 patients received alfuzosin hydrochloride extended-release tablets 10 mg daily. Table 4 provides the results of the three trials that evaluated the 10 mg dose.
There were two primary efficacy variables in these three studies. The International Prostate Symptom Score (IPSS, or AUA Symptom Score) consists of seven questions that assess the severity of both irritative (frequency, urgency, nocturia) and obstructive (incomplete emptying, stopping and starting, weak stream, and pushing or straining) symptoms, with possible scores ranging from 0 to 35 with higher numerical scores on the IPSS total symptom score representing greater severity of symptoms. The second efficacy variable was peak urinary flow rate. The peak flow rate was measured just prior to the next dose in study 2 and on average at 16 hours post-dosing in trials 1 and 3.
There was a statistically significant reduction from baseline to last assessment (Week 12) in the IPSS total symptom score versus placebo in all three studies, indicating a reduction in symptom severity (Table 5 and Figures 2, 3, and 4).
Table 4 — Mean Change (SD) from Baseline to week 12 in International Prostate Symptom Score in Three Randomized, Controlled, Double Blind Trials|
aDifference between baseline and week 12. | ||||||
|
Symptom****Score |
Trial****1 |
Trial****2 |
Trial****3 | |||
|
Placebo**** |
Alfuzosin**Hydrochloride** |
Placebo**** |
Alfuzosin**Hydrochloride** |
Placebo**** |
Alfuzosin**Hydrochloride** | |
|
Total symptom score |
|
|
|
|
|
|
|
Baseline**** |
18.2 (6.4)**** |
18.2 (6.3)**** |
17.7 (4.1)**** |
17.3 (3.5)**** |
17.7 (5.0)**** |
18.0 (5.4)**** |
|
Change a**** |
-1.6 (5.8)**** |
-3.6 (4.8)**** |
-4.9 (5.9)**** |
-6.9 (4.9)**** |
-4.6 (5.8)**** |
-6.5 (5.2)**** |
|
p-value**** |
0.001 |
0.002 |
0.007 |
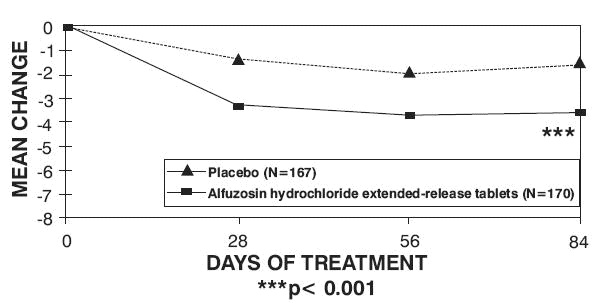
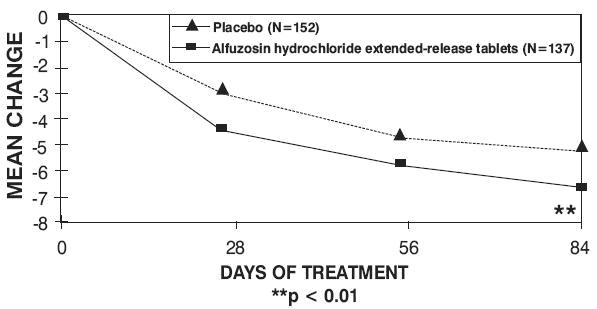
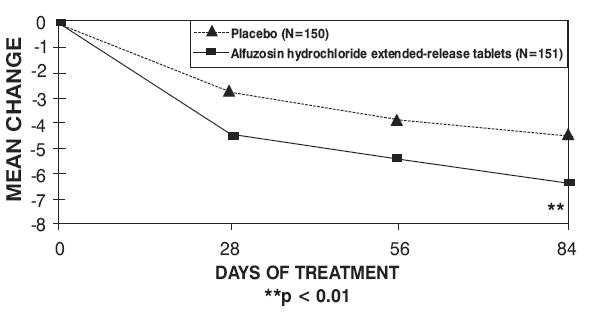
Peak urinary flow rate was increased statistically significantly from baseline to last assessment (Week 12) versus placebo in trials 1 and 2 (Table 5 and Figures 5, 6, and 7).
Table 5 — Mean (SD) Change from Baseline to Week 12 in Peak Urine Flow Rate (mL/sec) in Three Randomized, Controlled, Double-Blind Trials|
aDifference between baseline and week 12. | ||||||
|
Trial****1 |
Trial****2 |
Trial****3 | ||||
|
Placebo**** |
Alfuzosin**Hydrochloride** |
Placebo**** |
Alfuzosin**Hydrochloride** |
Placebo**** |
Alfuzosin**Hydrochloride** | |
|
MeanPeakflow****rate |
|
|
|
|
|
|
|
Baseline**** |
10.2 (4.0) |
9.9 (3.9) |
9.2 (2.0) |
9.4 (1.9) |
9.3 (2.6) |
9.5 (3.0) |
|
Change a**** |
0.2 (3.5) |
1.7 (4.2) |
1.4 (3.2) |
2.3 (3.6) |
0.9 (3.0) |
1.5 (3.3) |
|
p-value**** |
0.0004 |
0.03 |
0.22 |
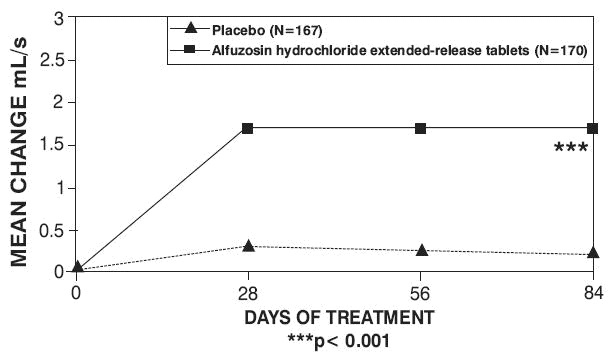
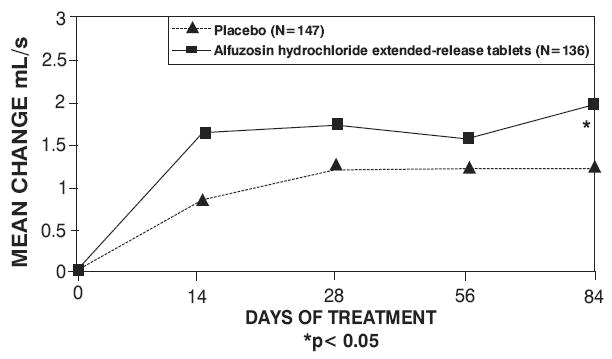
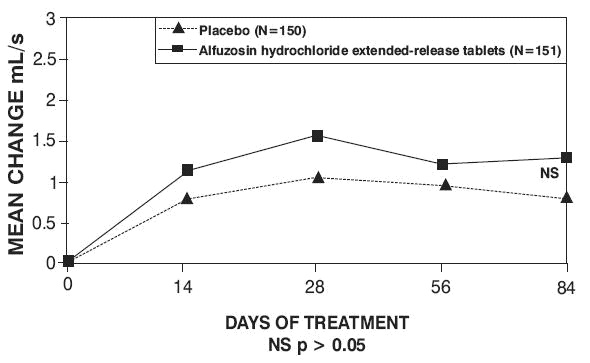
Mean total IPSS decreased at the first scheduled observation at Day 28 and mean peak flow rate increased starting at the first scheduled observation at Day 14 in trials 2 and 3 and Day 28 in trial 1.
