GS-441524
GS-441524
Approved
Approval ID
ed4122dd-66e4-47b1-9fa4-97fb53f3b67a
Product Type
BULK INGREDIENT - ANIMAL DRUG
Effective Date
Aug 27, 2025
Manufacturers
FDA
DARMERICA, LLC
DUNS: 080233052
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GS-441524
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
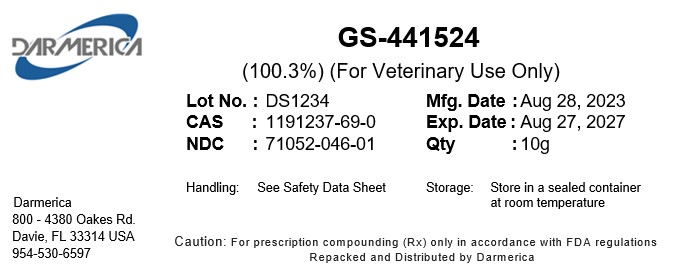
NDC Product Code71052-046
Product Classification
G
Generic Name
GS-441524
Product Specifications
Route of AdministrationNOT APPLICABLE
Effective DateAugust 27, 2025
FDA Product Classification
INGREDIENTS (1)
GS-441524Active
Quantity: 1 g in 1 g
Code: 1BQK176DT6
Classification: ACTIB
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 8/27/2025
GS-441524