Phentermine hydrochloride
These highlights do not include all the information needed to use Phentermine Hydrochloride Tablets and Capsules, USP 37.5 mg safely and effectively. See full prescribing information for Phentermine Hydrochloride Tablets and Capsules, USP 37.5 mg. PHENTERMINE hydrochloride capsules USP, for oral use CIVPHENTERMINE hydrochloride tablets USP, for oral use CIVInitial U.S. Approval: 1959
99c1325b-7152-481d-8a1f-6bf3e2c4808b
HUMAN PRESCRIPTION DRUG LABEL
Mar 15, 2024
Bryant Ranch Prepack
DUNS: 171714327
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
phentermine hydrochloride
PRODUCT DETAILS
INGREDIENTS (9)
Drug Labeling Information
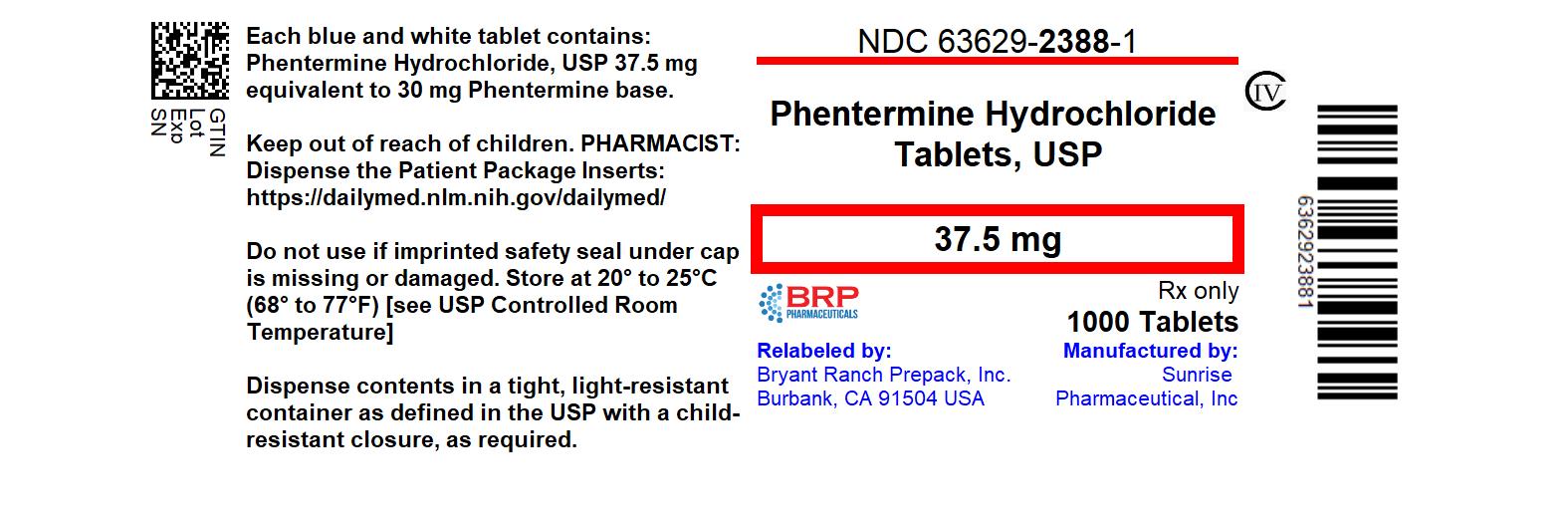
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Phentermine Hcl 37.5 mg Tab, #1000
INDICATIONS & USAGE SECTION
** 1 INDICATIONS AND USAGE**
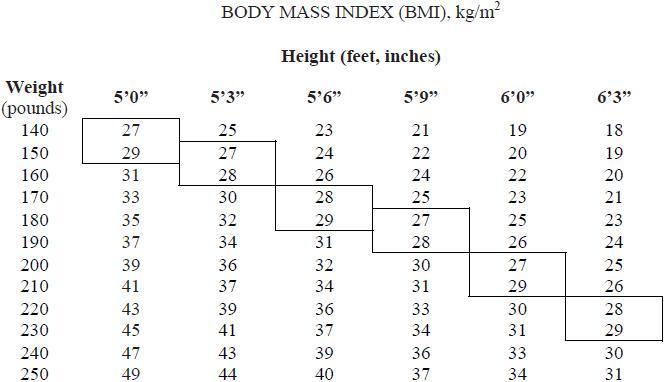
Phentermine hydrochloride is indicated as a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity for patients with an initial body mass index ≥ 30 kg/m2, or ≥ 27 kg/m2 in the presence of other risk factors (e.g., controlled hypertension, diabetes, hyperlipidemia).
Below is a chart of body mass index (BMI) based on various heights and weights.
BMI is calculated by taking the patient’s weight, in kilograms (kg), divided by the patient’s height, in meters (m), squared. Metric conversions are as follows: pounds ÷ 2.2 = kg; inches x 0.0254 = meters.
The limited usefulness of agents of this class, including phentermine, [see Clinical Pharmacology (12.1, 12.2)] should be measured against possible risk factors inherent in their use such as those described below.
Phentermine hydrochloride is a sympathomimetic amine anorectic indicated as a short-term adjunct (a few weeks) in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity for patients with an initial body mass index ≥ 30 kg/m2, or ≥ 27 kg/m2 in the presence of other risk factors (e.g., controlled hypertension, diabetes, hyperlipidemia). (1)
The limited usefulness of agents of this class, including Phentermine hydrochloride, should be measured against possible risk factors inherent in their use. (1)
CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
• History of cardiovascular disease (e.g., coronary artery disease, stroke,
arrhythmias, congestive heart failure, uncontrolled hypertension)
• During or within 14 days following the administration of monoamine oxidase
inhibitors
• Hyperthyroidism
• Glaucoma
• Agitated states
• History of drug abuse
• Pregnancy [see Use in Specific Populations (8.1)]
• Nursing [see Use in Specific Populations (8.3)]
• Known hypersensitivity, or idiosyncrasy to the sympathomimetic amines
- History of cardiovascular disease (e.g., coronary artery disease, stroke, arrhythmias, congestive heart failure, uncontrolled hypertension) (4)
- During or within 14 days following the administration of monoamine oxidase inhibitors (4)
- Hyperthyroidism (4)
- Glaucoma (4)
- Agitated states (4)
- History of drug abuse (4)
- Pregnancy (4, 8.1)
- Nursing (4, 8.3)
- Known hypersensitivity, or idiosyncrasy to the sympathomimetic amines (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
The following adverse reactions are described, or described in greater detail, in other sections:
• Primary pulmonary hypertension [see Warnings and Precautions (5.2)]
• Valvular heart disease [see Warnings and Precautions (5.3)]
• Effect on the ability to engage in potentially hazardous tasks [see Warnings and Precautions (5.5)]
• Withdrawal effects following prolonged high dosage administration [see Drug Abuse and Dependence (9.3)]
The following adverse reactions to phentermine have been identified:
Cardiovascular
Primary pulmonary hypertension and/or regurgitant cardiac valvular disease,
palpitation, tachycardia, elevation of blood pressure, ischemic events.
Central Nervous System
Overstimulation, restlessness, dizziness, insomnia, euphoria, dysphoria,
tremor, headache, psychosis.
Gastrointestinal
Dryness of the mouth, unpleasant taste, diarrhea, constipation, other
gastrointestinal disturbances.
Allergic
Urticaria.
Endocrine
Impotence, changes in libido.
Adverse events have been reported in the cardiovascular, central nervous, gastrointestinal, allergic, and endocrine systems. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Sunrise Pharmaceutical, Inc. at 732-382-6085 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch. (6)
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Monoamine Oxidase Inhibitors
Use of phentermine is contraindicated during or within 14 days following the administration of monoamine oxidase inhibitors because of the risk of hypertensive crisis.
7.2 Alcohol
Concomitant use of alcohol with phentermine may result in an adverse drug reaction.
7.3 Insulin and Oral Hypoglycemic Medications
Requirements may be altered [see Warnings and Precautions (5.9)].
7.4 Adrenergic Neuron Blocking Drugs
Phentermine may decrease the hypotensive effect of adrenergic neuron blocking drugs.
- Monoamine oxidase inhibitors: Risk of hypertensive crisis. (4, 7.1)
- Alcohol: Consider potential interaction (7.2)
- Insulin and oral hypoglycemics: Requirements may be altered. (7.3)
- Adrenergic neuron blocking drugs: Hypotensive effect may be decreased by phentermine. (7.4)
DOSAGE FORMS & STRENGTHS SECTION
3 DOSAGE FORMS AND STRENGTHS
Capsules containing 37.5 mg phentermine hydrochloride (equivalent to 30 mg
phentermine base).
Tablets with a functional score containing 37.5 mg phentermine hydrochloride
(equivalent to 30 mg phentermine base).
- Capsules containing 37.5 mg phentermine hydrochloride. (3)
- Tablets with a functional score containing 37.5 mg phentermine hydrochloride. (3)
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1Exogenous Obesity
Dosage should be individualized to obtain an adequate response with the lowest
effective dose.
The usual adult dose is one capsule (37.5 mg) daily as prescribed by the
physician, administered before breakfast or 1 to 2 hours after breakfast for
appetite control.
The usual adult dose is one tablet (37.5 mg) daily, as prescribed by the
physician, administered before breakfast or 1 to 2 hours after breakfast. The
dosage may be adjusted to the patient’s need. For some patients, half tablet
(18.75 mg) daily may be adequate, while in some cases it may be desirable to
give half tablets (18.75 mg) two times a day.
Phentermine is not recommended for use in pediatric patients ≤ 16 years of
age.
Late evening medication should be avoided because of the possibility of
resulting insomnia.
2.2 Dosage in Patients With Renal Impairment
The recommended maximum dosage of phentermine hydrochloride is 15 mg daily for patients with severe renal impairment (eGFR 15 to 29 mL/min/1.73m2). Avoid use of phentermine hydrochloride in patients with eGFR less than 15 mL/min/1.73m2 or end-stage renal disease requiring dialysis [see Use in Specific Populations(8.6) and Clinical Pharmacology (12.3)].
- Dosage should be individualized to obtain an adequate response with the lowest effective dose. (2.1)
- Late evening administration should be avoided (risk of insomnia). (2.1)
- Phentermine hydrochloride can be taken with or without food. (2.1)
- Limit the dosage to 15 mg daily for patients with severe renal impairment (eGFR 15 to 29 mL/min/1.73m2)- (2.2)
USE IN SPECIFIC POPULATIONS SECTION
8 USE IN SPECIFIC POPULATIONS
** 8.1 Pregnancy**
Teratogenic Effects
Pregnancy category X
Phentermine is contraindicated during pregnancy because weight loss offers no
potential benefit to a pregnant woman and may result in fetal harm. A minimum
weight gain, and no weight loss, is currently recommended for all pregnant
women, including those who are already overweight or obese, due to obligatory
weight gain that occurs in maternal tissues during pregnancy. Phentermine has
pharmacologic activity similar to amphetamine (d- and dll-amphetamine) [see Clinical Pharmacology(12.1)]. Animal reproduction studies have not been
conducted with phentermine. If this drug is used during pregnancy, or if the
patient becomes pregnant while taking this drug, the patient should be
apprised of the potential hazard to a fetus.
8.3 Nursing Mothers
It is not known if phentermine is excreted in human milk; however, other amphetamines are present in human milk. Because of the potential for serious adverse reactions in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
8.4 Pediatric Use
Safety and effectiveness in pediatric patients have not been established. Because pediatric obesity is a chronic condition requiring long-term treatment, the use of this product, approved for short-term therapy, is not recommended.
8.5 Geriatric Use
In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
8.6 Renal Impairment
Based on the reported excretion of phentermine in urine, exposure increases can be expected in patients with renal impairment [see Clinical Pharmacology (12.3)].
Use caution when administering phentermine to patients with renal impairment. In patients with severe renal impairment (eGFR 15 to 29 mL/min/1.73m2), limit the dosage of phentermine to 15 mg daily [see Dosage and Administration (2.2)]. Phentermine has not been studied in patients with eGFR less than 15 mL/min/m2, including end-stage renal disease requiring dialysis; avoid use in these populations.
- Nursing mothers: Discontinue drug or nursing taking into consideration importance of drug to mother. (4, 8.3)
- Pediatric use: Safety and effectiveness not established. (8.4)
- Geriatric use: Due to substantial renal excretion, use with caution. (8.5)
- Renal Impairment: Avoid use in patients with eGFR less than 15 mL/min/m2 or end-stage renal disease requiring dialysis. (8.6)
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Phentermine is a Schedule IV controlled substance.
9.2 Abuse
Phentermine is related chemically and pharmacologically to the amphetamines. Amphetamines and other stimulant drugs have been extensively abused and the possibility of abuse of phentermine should be kept in mind when evaluating the desirability of including a drug as part of a weight reduction program.
9.3 Dependence
Abuse of amphetamines and related drugs may be associated with intense psychological dependence and severe social dysfunction. There are reports of patients who have increased the dosage of these drugs to many times than recommended. Abrupt cessation following prolonged high dosage administration results in extreme fatigue and mental depression; changes are also noted on the sleep EEG. Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. A severe manifestation of chronic intoxication is psychosis, often clinically indistinguishable from schizophrenia.
DESCRIPTION SECTION
11 DESCRIPTION
Phentermine hydrochloride USP is a sympathomimetic amine anorectic. It has the chemical name of α,α,-Dimethylphenethylamine hydrochloride. The structural formula is as follows:
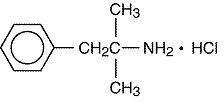
C10H15N•HCl M.W. 185.7
Phentermine hydrochloride is a white, odorless, hygroscopic, crystalline powder which is soluble in water and lower alcohols, slightly soluble in chloroform and insoluble in ether.
Phentermine hydrochloride, an anorectic agent for oral administration, is available as a capsule or tablet containing 37.5 mg of phentermine hydrochloride (equivalent to 30 mg of phentermine base).
Phentermine hydrochloride capsules, USP 37.5 mg contain the inactive ingredients Colloidal Silicon Dioxide, Corn Starch, FD&C Blue # 1, FD&C Red # 3, FD&C Yellow # 6, Gelatin, Lactose Monohydrate, Magnesium Stearate, and Titanium Dioxide. The ingredients in the black imprinting ink are shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide and black iron oxide.
Phentermine hydrochloride tablets, USP 37.5 mg contain the inactive ingredients Colloidal Silicon Dioxide, Corn Starch, FD&C Blue # 1, Lactose Monohydrate, Magnesium Stearate, Microcrystalline Cellulose, Pregelatinized Starch (botanical source: maize), Sugar Spheres, and Stearic Acid.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Phentermine is a sympathomimetic amine with pharmacologic activity similar to the prototype drugs of this class used in obesity, amphetamine (d- and dll- amphetamine). Drugs of this class used in obesity are commonly known as “anorectics” or “anorexigenics.” It has not been established that the primary action of such drugs in treating obesity is one of appetite suppression since other central nervous system actions, or metabolic effects, may also be involved.
12.2 Pharmacodynamics
Typical actions of amphetamines include central nervous system stimulation and elevation of blood pressure. Tachyphylaxis and tolerance have been demonstrated with all drugs of this class in which these phenomena have been looked for.
12.3 Pharmacokinetics
Following the administration of phentermine, phentermine reaches peak
concentrations (Cmax) after 3 to 4.4 hours.
Specific Populations
Renal Impairment
Cumulative urinary excretion of phentermine under uncontrolled urinary pH
conditions was 62% to 85%.
Systemic exposure of phentermine may increase up to 91%, 45%, and 22% in
patients with severe, moderate, and mild renal impairment, respectively [see Dosage and Administration (2.2) and Use in Specific Populations (8.6)].
Drug Interactions
In a single- dose study comparing the exposures after oral administration of a
combination capsule of 15 mg phentermine and 92 mg topiramate to the exposures
after oral administration of a 15 mg phentermine capsule or a 92 mg topiramate
capsule, there is no significant topiramate exposure change in the presence of
phentermine. However in the presence of topiramate, phentermine Cmax and AUC
increase 13% and 42%, respectively.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies have not been performed with phentermine to determine the potential for carcinogenesis, mutagenesis or impairment of fertility.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
In relatively short-term clinical trials, adult obese subjects instructed in dietary management and treated with “anorectic” drugs lost more weight on the average than those treated with placebo and diet.
The magnitude of increased weight loss of drug-treated patients over placebo- treated patients is only a fraction of a pound a week. The rate of weight loss is greatest in the first weeks of therapy for both drug and placebo subjects and tends to decrease in succeeding weeks. The possible origins of the increased weight loss due to the various drug effects are not established. The amount of weight loss associated with the use of an “anorectic” drug varies from trial to trial, and the increased weight loss appears to be related in part to variables other than the drugs prescribed, such as the physician- investigator, the population treated and the diet prescribed. Studies do not permit conclusions as to the relative importance of the drug and non-drug factors on weight loss.
The natural history of obesity is measured over several years, whereas the studies cited are restricted to a few weeks’ duration; thus, the total impact of drug-induced weight loss over that of diet alone must be considered clinically limited.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Available in tablets containing 37.5 mg phentermine hydrochloride (equivalent to 30 mg phentermine base). Each white with blue specks, capsule shaped tablet is debossed with “N” on the left side of bisect and “4” on the right side of bisect on one side and plain on the other side, having a functional score.
Tablets are packaged in bottles of 1,000 (NDC 63629-2388-1)
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
Dispense in a tight container as defined in the USP, with a child-resistant closure (as required).
KEEP THIS AND ALL MEDICATIONS OUT OF THE REACH OF THE CHILDREN.
Repackaged/Relabeled by:
Bryant Ranch Prepack, Inc.
Burbank, CA 91504
OVERDOSAGE SECTION
10 OVERDOSAGE
The least amount feasible should be prescribed or dispensed at one time in order to minimize the possibility of overdosage.
10.1 Acute Overdosage
Manifestations of acute overdosage include restlessness, tremor, hyperreflexia, rapid respiration, confusion, assaultiveness, hallucinations, and panic states. Fatigue and depression usually follow the central stimulation. Cardiovascular effects include tachycardia, arrhythmia, hypertension or hypotension, and circulatory collapse. Gastrointestinal symptoms include nausea, vomiting, diarrhea and abdominal cramps. Overdosage of pharmacologically similar compounds has resulted in fatal poisoning and usually terminates in convulsions and coma.
Management of acute phentermine hydrochloride intoxication is largely symptomatic and includes lavage and sedation with a barbiturate. Experience with hemodialysis or peritoneal dialysis is inadequate to permit recommendations in this regard. Acidification of the urine increases phentermine excretion. Intravenous phentolamine (Regitine®, CIBA) has been suggested on pharmacologic grounds for possible acute, severe hypertension, if this complicates overdosage.
10.2 Chronic Intoxication
Manifestations of chronic intoxication with anorectic drugs include severe dermatoses, marked insomnia, irritability, hyperactivity and personality changes. The most severe manifestation of chronic intoxications is psychosis, often clinically indistinguishable from schizophrenia. See Drug Abuse and Dependence (9.3).
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Patients must be informed that Phentermine hydrochloride is a short-term (a few weeks) adjunct in a regimen of weight reduction based on exercise, behavioral modification and caloric restriction in the management of exogenous obesity, and that coadministration of phentermine with other drugs for weight loss is not recommended [see Indications and Usage (1) and Warnings and Precautions(5)].
Patients must be instructed on how much phentermine to take, and when and how to take it [see Dosage and Administration (2)].
Advise pregnant women and nursing mothers not to use phentermine [see Use in Specific Populations (8.1, 8.3)].
Patients must be informed about the risks of use of phentermine (including the
risks discussed in Warnings and Precautions), about the symptoms of potential
adverse reactions and when to contact a physician and/or take other action.
The risks include, but are not limited to:
• Development of primary pulmonary hypertension [see Warnings and Precautions (5.2)]
• Development of serious valvular heart disease [see Warnings and Precautions (5.3)]
• Effects on the ability to engage in potentially hazardous tasks [see Warnings and Precautions (5.5)]
• The risk of an increase in blood pressure [see Warnings and Precautions (5.8) and Adverse Reactions (6)]
• The risk of interactions [see Contraindications (4), Warnings and Precautions (5) and Drug Interactions (7)]
See also, for example, Adverse Reactions (6) and Use in Specific Populations (8).
The patients must also be informed about
• the potential for developing tolerance and actions if they suspect
development of tolerance [see Warnings and Precautions (5.4)] and
• the risk of dependence and the potential consequences of abuse [see Warnings and Precautions (5.6), Drug Abuse and Dependence (9), and Overdosage (10)].
Tell patients to keep phentermine in a safe place to prevent theft, accidental overdose, misuse or abuse. Selling or giving away phentermine may harm others and is against the law.
All trademarks are the property of their respective owners.
Rx Only
Manufactured & Distributed By:
Sunrise Pharmaceutical, Inc.
Rahway, New Jersey 07065
Revised: 04/2017
5097/03
