DISOPYRAMIDE PHOSPHATE
DISOPYRAMIDE PHOSPHATE CAPSULES, USP
7d550b12-065a-416c-93fa-b61368eeb36f
HUMAN PRESCRIPTION DRUG LABEL
Jul 6, 2023
Mayne Pharma Commercial LLC
DUNS: 867220261
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
DISOPYRAMIDE PHOSPHATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (10)
DISOPYRAMIDE PHOSPHATE
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (12)
Drug Labeling Information
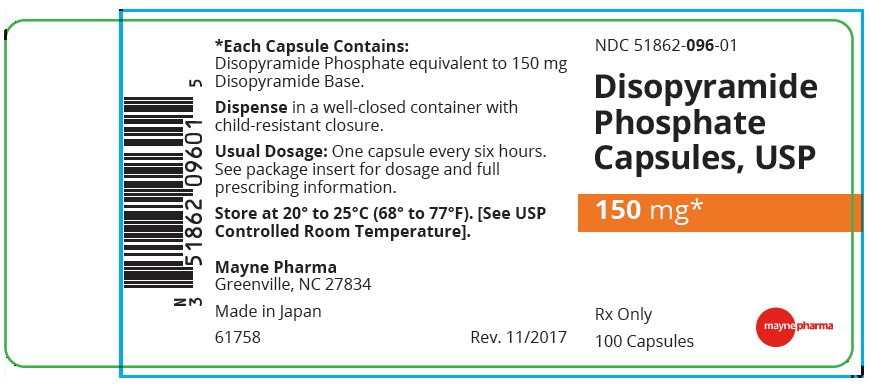
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 150 mg Capsule Bottle Label
NDC 51862-096-01
Disopyramide
Phosphate
Capsules, USP
150 mg*
Rx Only
100 Capsules
mayne pharma
OVERDOSAGE SECTION
OVERDOSAGE
Symptoms
Deliberate or accidental overdosage of oral disopyramide may be followed by apnea, loss of consciousness, cardiac arrhythmias, and loss of spontaneous respiration. Death has occurred following overdosage.
Toxic plasma levels of disopyramide produce excessive widening of the QRS complex and Q-T interval, worsening of congestive heart failure, hypotension, varying kinds and degrees of conduction disturbance, bradycardia, and finally asystole. Obvious anticholinergic effects are also observed.
The approximate oral LD 50of disopyramide phosphate is 580 and 700 mg/kg for rats and mice, respectively.
Treatment
Experience indicates that prompt and vigorous treatment of overdosage is necessary, even in the absence of symptoms. Such treatment may be life-saving. No specific antidote for disopyramide phosphate has been identified. Treatment should be symptomatic and may include induction of emesis or gastric lavage, administration of a cathartic followed by activated charcoal by mouth or stomach tube, intravenous administration of isoproterenol and dopamine, insertion of an intra-aortic balloon for counterpulsation, and mechanically assisted ventilation. Hemodialysis or, preferably, hemoperfusion with charcoal may be employed to lower serum concentration of the drug.
The electrocardiogram should be monitored, and supportive therapy with cardiac glycosides and diuretics should be given as required.
If progressive AV block should develop, endocardial pacing should be implemented. In case of any impaired renal function, measures to increase the glomerular filtration rate may reduce the toxicity (disopyramide is excreted primarily by the kidney).
The anticholinergic effects can be reversed with neostigmine at the discretion of the physician.
Altering the urinary pH in humans does not affect the plasma half-life or the amount of disopyramide excreted in the urine.
HOW SUPPLIED SECTION
HOW SUPPLIED
Disopyramide Phosphate Capsules (equivalent to 100 mg disopyramide base) are opaque orange capsules imprinted with m on one side and 095 on the other. Supplied in bottles of 100.
Disopyramide Phosphate Capsules (equivalent to 150 mg disopyramide base) are opaque brown capsules imprinted with m on one side and 096 on the other. Supplied in bottles of 100.
Dispense in a well-closed container with child-resistant closure.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
