Azilect
These highlights do not include all the information needed to use safely and effectively. See full prescribing information for . Initial U.S. Approval: 2006
a40d0e73-3f9f-4b01-979d-402c9cdaeb60
HUMAN PRESCRIPTION DRUG LABEL
Jun 30, 2020
Teva Neuroscience, Inc.
DUNS: 009906397
Products 2
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Rasagiline mesylate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Rasagiline mesylate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel
NDC 68546-229-56
AZILECT®(rasagiline tablets)
1 mg
****30 Tablets Rx only

DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Meperidine
Serious, sometimes fatal reactions have been precipitated with concomitant use of meperidine (e.g., Demerol and other tradenames) and MAO inhibitors including selective MAO-B inhibitors [see Contraindications (4)].
7.2 Dextromethorphan
The concomitant use of AZILECT and dextromethorphan was not allowed in clinical studies. The combination of MAO inhibitors and dextromethorphan has been reported to cause brief episodes of psychosis or bizarre behavior. Therefore, in view of AZILECT’s MAO inhibitory activity, dextromethorphan is contraindicated for use with AZILECT [see Contraindications (4)].
7.3 MAO Inhibitors
AZILECT is contraindicated for use with other MAO inhibitors because of the increased risk of nonselective MAO inhibition that may lead to a hypertensive crisis [see Contraindications (4)].
7.4 Sympathomimetic Medications
The concomitant use of AZILECT and sympathomimetic medications was not allowed in clinical studies. Severe hypertensive reactions have followed the administration of sympathomimetics and nonselective MAO inhibitors. Hypertensive crisis has been reported in patients taking the recommended dose of AZILECT and sympathomimetic medications. Severe hypertension has been reported in patients taking the recommended dose of AZILECT and ophthalmic drops containing sympathomimetic medications.
Because AZILECT is a selective MAOI, hypertensive reactions are not ordinarily expected with the concomitant use of sympathomimetic medications. Nevertheless, caution should be exercised when concomitantly using recommended doses of AZILECT with any sympathomimetic medications including nasal, oral, and ophthalmic decongestants and cold remedies.
7.5 Antidepressants
Concomitant use of AZILECT with one of many classes of antidepressants (e.g., SSRIs, SNRIs, triazolopyridine, tricyclic, or tetracyclic antidepressants) is not recommended [see Warnings and Precautions (5.2) and Clinical Pharmacology (12.3)]. Concomitant use of AZILECT and MAO inhibitors is contraindicated [see Contraindications (4)].
7.6 Ciprofloxacin or Other CYP1A2 Inhibitors
Rasagiline plasma concentrations may increase up to 2 fold in patients using concomitant ciprofloxacin and other CYP1A2 inhibitors. This could result in increased adverse events. Patients taking concomitant ciprofloxacin or other CYP1A2 inhibitors should not exceed a dose of AZILECT 0.5 mg once daily [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
7.7 Tyramine/Rasagiline Interaction
MAO in the gastrointestinal tract and liver (primarily type A) provides protection from exogenous amines (e.g., tyramine) that have the capacity, if absorbed intact, to cause a tyramine reaction with hypertension including clinical syndromes referred to as hypertensive urgency, crisis, or emergency. Foods and medications containing large amounts of exogenous amines (e.g., from fermented cheese, herring, over-the-counter cough/cold medications) may cause release of norepinephrine resulting in a rise in systemic blood pressure.
Results of a special tyramine challenge study indicate that rasagiline is selective for MAO-B at recommended doses and can be used without dietary tyramine restriction. However, certain foods may contain very high amounts (i.e., 150 mg or greater) of tyramine and could potentially cause a hypertensive reaction in individual patients taking AZILECT due to increased sensitivity to tyramine. Selectivity for inhibiting MAO-B diminishes in a dose-related manner as the dose is progressively increased above the recommended daily doses.
There were no cases of hypertensive crisis in the clinical development program associated with 1 mg daily AZILECT treatment, in which most patients did not follow dietary tyramine restriction.
There have been postmarketing reports of patients who experienced significantly elevated blood pressure (including rare cases of hypertensive crisis) after ingestion of unknown amounts of tyramine-rich foods while taking recommended doses of AZILECT. Patients should be advised to avoid foods containing a very large amount of tyramine while taking recommended doses of AZILECT [see Warnings and Precautions (5.1)].
7.8 Dopaminergic Antagonists
It is possible that dopamine antagonists, such as antipsychotics or metoclopramide, could diminish the effectiveness of AZILECT.
- Meperidine: Risk of serotonin syndrome (4, 7.1)
- Dextromethorphan: Risk of psychosis or bizarre behavior (4, 7.2)
- MAO inhibitors: Risk of non-selective MAO inhibition and hypertensive crisis (4, 7.3)
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
The effectiveness of AZILECT for the treatment of Parkinson’s disease was established in four 18- to 26-week, randomized, placebo-controlled trials, as initial monotherapy or adjunct therapy.
14.1 Monotherapy Use of AZILECT
Study 1 was a double-blind, randomized, fixed-dose parallel group, 26-week study in early Parkinson’s disease patients not receiving any concomitant dopaminergic therapy at the start of the study. The majority of the patients were not treated with medications for Parkinson’s disease before receiving AZILECT.
In Study 1, 404 patients were randomly assigned to receive placebo (138 patients), AZILECT 1 mg/day (134 patients) or AZILECT 2 mg/day (132 patients). Patients were not allowed to take levodopa, dopamine agonists, selegiline, or amantadine, but could take stable doses of anticholinergic medication, if necessary. The average Parkinson’s disease duration was approximately 1 year (range 0 to 11 years).
The primary measure of effectiveness was the change from baseline in the total score of the Unified Parkinson’s Disease Rating Scale (UPDRS), [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)]. The UPDRS is a multi-item rating scale that measures the ability of a patient to perform mental and motor tasks as well as activities of daily living. A reduction in the score represents improvement and a beneficial change from baseline appears as a negative number.
AZILECT (1 or 2 mg once daily) was superior to placebo on the primary measure of effectiveness in patients receiving six months of treatment and not on dopaminergic therapy. The effectiveness of AZILECT 1 mg and 2 mg was comparable. Table 4 shows the results of Study 1. There were no differences in effectiveness based on age or gender between AZILECT 1 mg/day and placebo.
Table 4: Change in Total UPDRS Score in Study 1|
Baseline score |
Change from baseline to termination score |
p-value vs. placebo | |
|
Placebo |
24.5 |
3.9 |
--- |
|
AZILECT 1 mg |
24.7 |
0.1 |
0.0001 |
|
AZILECT 2 mg |
25.9 |
0.7 |
0.0001 |
14.2 Adjunct Use of AZILECT
Study 2 was a double-blind, randomized, placebo-controlled, parallel group, 18-week study, investigating AZILECT 1 mg as adjunct therapy to dopamine agonists without levodopa. Patients were on a stable dose of dopamine agonist (ropinirole, mean 8 mg/day or pramipexole, mean 1.5 mg/day) therapy for ≥ 30 days, but at doses not sufficient to control Parkinson’s disease symptoms.
In Study 2, 321 patients randomly received placebo (162 patients) or AZILECT 1 mg/day (159 patients) and had a post-baseline assessment. The average Parkinson’s disease duration was approximately 2 years (range 0.1 to 14.5 years).
The primary measure of effectiveness was the change from baseline in the total score of the Unified Parkinson’s Disease Rating Scale (UPDRS) [mentation (Part I) + activities of daily living (ADL) (Part II) + motor function (Part III)].
In Study 2, AZILECT 1 mg was superior to placebo on the primary measure of effectiveness (see Table 5).
Table 5: Change in Total UPDRS Score in Study 2|
Baseline score |
Change from baseline to termination score* |
p-value vs. placebo | |
|
Placebo |
29.8 |
–1.2 |
--- |
|
AZILECT1 mg |
32.1 |
–3.6 |
0.012 |
*A negative change from baseline indicates improvement in the UPDRS
Secondary outcome assessment of the individual subscales of the UPDRS indicates that the UPDRS Part III motor subscale was primarily responsible for the overall AZILECT effect on the UPDRS score (see Table 6).
Table 6: Secondary Measures of Effectiveness in Study 2|
Baseline (score) |
Change from baseline to termination score | |
|
UPDRS Part II ADL (Activities of Daily Living) subscale score | ||
|
Placebo |
7.9 |
0.4 |
|
AZILECT 1 mg |
8.6 |
-0.3 |
|
UPDRS Part III Motor subscale score | ||
|
Placebo |
20.4 |
-1.2 |
|
AZILECT 1 mg |
22.2 |
-3.7 |
Study 3 and Study 4 were randomized, multinational trials conducted in more advanced Parkinson’s disease patients treated chronically with levodopa and experiencing motor fluctuations (including but not limited to, end of dose “wearing off,” sudden or random “off,” etc.). Study 3 was conducted in North America (U.S. and Canada) and compared AZILECT 0.5 mg and 1 mg daily to placebo. Study 4 was conducted outside of North America in Europe, Argentina, and Israel, and compared AZILECT 1 mg daily to placebo.
Patients had Parkinson’s disease for an average of 9 years (range 5 months to 33 years), had taken levodopa for an average of 8 years (range 5 months to 32 years), and had motor fluctuations for approximately 3 to 4 years (range 1 month to 23 years). Patients kept home Parkinson’s disease diaries just prior to baseline and at specified intervals during the trial. Diaries recorded one of the following four conditions for each half-hour interval over a 24-hour period: “ON” (period of relatively good function and mobility) as either “ON” with no dyskinesia or without troublesome dyskinesia, or “ON” with troublesome dyskinesia, “OFF” (period of relatively poor function and mobility) or asleep. “Troublesome” dyskinesia is defined as dyskinesia that interferes with the patient’s daily activity. All patients had inadequate control of their motor symptoms with motor fluctuations typical of advanced stage disease despite receiving levodopa/decarboxylase inhibitor. The average dose of levodopa taken with a decarboxylase inhibitor was approximately 700 to 800 mg (range 150 to 3000 mg/day). Patients continued their stable doses of additional anti-PD medications at entry into the trials. Approximately 65% of patients in both studies were also taking a dopamine agonist. In the North American study (Study 3), approximately 35% of patients took entacapone with levodopa/decarboxylase inhibitor. The majority of patients taking entacapone were also taking a dopamine agonist.
In Study 3 and Study 4, the primary measure of effectiveness was the change in the mean number of hours spent in the “OFF” state at baseline compared to the mean number of hours spent in the “OFF” state during the treatment period.
In Study 3, patients were randomly assigned to receive placebo (159 patients), AZILECT 0.5 mg/day (164 patients), or AZILECT 1 mg/day (149 patients) for 26 weeks. Patients averaged 6 hours daily in the “OFF” state at baseline as confirmed by home diaries.
In Study 4, patients were randomly assigned to receive placebo (229 patients), AZILECT 1 mg/day (231 patients) or a COMT inhibitor (active comparator), taken along with scheduled doses of levodopa/decarboxylase inhibitor (227 patients) for 18 weeks. Patients averaged 5.6 hours daily in the “OFF” state at baseline as confirmed by home diaries.
In Study 3 and Study 4, AZILECT 1 mg once daily reduced “OFF” time compared to placebo when added to levodopa in patients experiencing motor fluctuations (Tables 7 and 8). The lower dose (0.5 mg) of AZILECT also significantly reduced “OFF” time (Table 7), but had a numerically smaller effect than the 1 mg dose of AZILECT. In Study 4, the active comparator also reduced “OFF” time when compared to placebo.
Table 7: Change in mean total daily “OFF” time in Study 3|
Baseline (hours) |
Change from baseline to treatment period (hours) |
p-value vs. placebo | |
|
Placebo |
6.0 |
-0.9 |
--- |
|
AZILECT 0.5 mg |
6.0 |
-1.4 |
0.0199 |
|
AZILECT 1.0 mg |
6.3 |
-1.9 |
< 0.0001 |
|
Baseline (hours) |
Change from baseline to treatment period (hours) |
p-value vs. placebo | |
|
Placebo |
5.5 |
- 0.40 |
--- |
|
AZILECT 1.0 mg |
5.6 |
-1.2 |
0.0001 |
In Study 3 and Study 4, dose reduction of levodopa was allowed within the first 6 weeks, if dopaminergic side effects developed including dyskinesia or hallucinations. In Study 3, the levodopa dose was reduced in 8% of patients in the placebo group and in 16% and 17% of patients in the 0.5 mg/day and 1 mg/day AZILECT groups, respectively. When levodopa was reduced, the dose was reduced by 7%, 9%, and 13% in the placebo, 0.5 mg/day, and 1 mg/day groups, respectively. In Study 4, levodopa dose reduction occurred in 6% of patients in the placebo group and in 9% in the AZILECT 1 mg/day groups, respectively. When levodopa was reduced, it was reduced by 13% and 11% in the placebo and the AZILECT groups, respectively.
There were no differences in effectiveness based on age or gender between AZILECT 1 mg/day and placebo.
Several secondary outcome assessments in the two studies showed statistically significant improvements with rasagiline. These included effects on the activities of daily living (ADL) subscale of the UPDRS performed during an “OFF” period and the motor subscale of the UPDRS performed during an “ON” period. In both scales, a negative response represents improvement. Tables 9 and 10 show these results for Studies 3 and 4.
Table 9: Secondary Measures of Effectiveness in Study 3|
Baseline (score) |
Change from baseline to last value | |
|
UPDRS ADL (Activities of Daily Living) subscale score while “OFF” | ||
|
Placebo |
15.5 |
0.68 |
|
AZILECT 0.5 mg |
15.8 |
-0.60 |
|
AZILECT 1 mg |
15.5 |
-0.68 |
|
UPDRS Motor subscale score while “ON” | ||
|
Placebo |
20.8 |
1.21 |
|
AZILECT 0.5 mg |
21.5 |
-1.43 |
|
AZILECT 1 mg |
20.9 |
-1.30 |
|
Baseline (score) |
Change from baseline to last value | |
|
UPDRS ADL (Activities of Daily Living) subscale score while “OFF” | ||
|
Placebo |
18.7 |
-0.89 |
|
AZILECT 1 mg |
19.0 |
-2.61 |
|
UPDRS Motor subscale score while “ON” | ||
|
Placebo |
23.5 |
-0.82 |
|
AZILECT 1 mg |
23.8 |
-3.87 |
DESCRIPTION SECTION
11 DESCRIPTION
AZILECT® tablets contain rasagiline (as the mesylate), a propargylamine-based drug indicated for the treatment of idiopathic Parkinson’s disease. Rasagiline mesylate is designated chemically as: 1H-Inden-1-amine, 2, 3-dihydro-N-2-propynyl-, (1R)-, methanesulfonate. The empirical formula of rasagiline mesylate is C12H13N•CH4SO3 and its molecular weight is 267.34.
Its structural formula is:
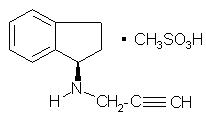
Rasagiline mesylate is a white to off-white powder, freely soluble in water or ethanol and sparingly soluble in isopropanol. Each AZILECT tablet for oral administration contains 0.5 mg or 1 mg of rasagiline (equivalent to 0.78 mg or 1.56 mg of rasagiline mesylate).
Each AZILECT tablet also contains the following inactive ingredients: mannitol, starch, pregelatinized starch, colloidal silicon dioxide, stearic acid, and talc.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Hypertension
Advise patients that treatment with recommended doses of AZILECT may be associated with elevations of blood pressure. Tell patients who experience elevation of blood pressure while taking AZILECT to contact their healthcare provider.
The risk of using higher than recommended daily doses of AZILECT should be explained, and a brief description of the tyramine associated hypertensive reaction provided.
Advise patients to avoid certain foods (e.g., aged cheese) containing a very large amount of tyramine while taking recommended doses of AZILECT because of the potential for large increases in blood pressure. If patients eat foods very rich in tyramine and do not feel well soon after eating, they should contact their healthcare provider [see Warnings and Precautions (5.1)].
Serotonin Syndrome
Tell patients to inform their physician if they are taking, or planning to take, any prescription or over-the-counter drugs, especially antidepressants and over-the-counter cold medications, since there is a potential for interaction with AZILECT. Because patients should not use meperidine or certain other analgesics with AZILECT, they should contact their healthcare provider before taking analgesics [see Contraindications (4) and Warnings and Precautions (5.2)].
Falling Asleep During Activities of Daily Living and Somnolence
Advise and alert patients about the potential for sedating effects associated with AZILECT and other dopaminergic medications, including somnolence and particularly to the possibility of falling asleep while engaged in activities of daily living. Because somnolence can be a frequent adverse reaction with potentially serious consequences, patients should neither drive a car nor engage in other potentially dangerous activities until they have gained sufficient experience with AZILECT and other dopaminergic medications to gauge whether or not it affects their mental and/or motor performance adversely. Advise patients that if increased somnolence or new episodes of falling asleep during activities of daily living (e.g., watching television, passenger in a car, etc.) are experienced at any time during treatment, they should not drive or participate in potentially dangerous activities until they have contacted their physician. Patients should not drive, operate machinery, or work at heights during treatment if they have previously experienced somnolence and/or have fallen asleep without warning prior to use of AZILECT.
Because of possible additive effects, advise patients to exercise caution when patients are taking other sedating medications, alcohol, or other central nervous system depressants (e.g., benzodiazepines, antipsychotics, antidepressants) in combination with AZILECT or when taking concomitant medications that increase plasma levels of rasagiline (e.g., ciprofloxacin) [see Warnings and Precautions (5.3)].
Ciprofloxacin or Other CYP1A2 Inhibitors
Inform patients that they should contact their healthcare provider of AZILECT if they take ciprofloxacin or a similar drug that could increase blood levels of rasagiline because of the need to adjust the dose of AZILECT [see Dosage and Administration (2.2) and Warnings and Precautions (5.4)].
Hepatic Impairment
Tell patients who have hepatic problems to contact their healthcare provider regarding possible changes in AZILECT dosing [see Warnings and Precautions (5.5)].
Hypotension / Orthostatic Hypotension
Patients should be advised that they may develop orthostatic hypotension with or without symptoms such as dizziness, nausea, syncope, and sometimes sweating. Hypotension and/or orthostatic symptoms may occur more frequently during initial therapy or with an increase in dose at any time (cases have been seen after weeks of treatment). Accordingly, patients should be cautioned against standing up rapidly after sitting or lying down, especially if they have been doing so for prolonged periods, and especially, at the initiation of treatment with AZILECT [see Warnings and Precautions (5.6)].
Dyskinesia
Advise patients taking AZILECT as adjunct to levodopa that there is a possibility of dyskinesia or increased dyskinesia [see Warnings and Precautions (5.7)].
Hallucinations / Psychotic-Like Behavior
Inform patients that hallucinations or other manifestations of psychotic-like behavior can occur when taking AZILECT. Advise patients that, if they have a major psychotic disorder, that AZILECT should not ordinarily be used because of the risk of exacerbating the psychosis. Patients with a major psychotic disorder should also be aware that many treatments for psychosis may decrease the effectiveness of AZILECT [see Warnings and Precautions (5.8)].
Impulse Control/Compulsive Behaviors
Advise patients that they may experience intense urges to gamble, increased sexual urges, other intense urges, and the inability to control these urges while taking one or more of the medications that increase central dopaminergic tone and that are generally used for the treatment of Parkinson’s disease (including AZILECT). Although it is not proven that the medications caused these events, these urges were reported to have stopped in some cases when the dose was reduced or the medication was stopped. Prescribers should ask patients about the development of new or increased gambling urges, sexual urges, or other urges while being treated with AZILECT. Patients should inform their physician if they experience new or increased gambling urges, increased sexual urges, or other intense urges while taking AZILECT. Physicians should consider dose reduction or stopping the medication if a patient develops such urges while taking AZILECT [see Warnings and Precautions (5.9)].
Withdrawal-Emergent Hyperpyrexia and Confusion
Tell patients to contact their healthcare provider if they wish to discontinue AZILECT [see Warnings and Precautions (5.10)].
Missing Dose
Instruct patients to take AZILECT as prescribed. If a dose is missed, the patient should not double-up the dose of AZILECT. The next dose should be taken at the usual time on the following day.
Pregnancy
Advise patients to notify their healthcare provider if they are pregnant or plan to become pregnant [see Use in Specific Populations (8.1)].

AZI-004
Distributed by: Teva Pharmaceuticals USA, Inc., Parsippany, NJ 07054
©2020 Teva Neuroscience, Inc.
