Rebif
These highlights do not include all the information needed to use REBIF safely and effectively. See full prescribing information for REBIF. REBIF (interferon beta-1a), for subcutaneous injection Initial U.S. Approval: 1996
c6fcb5d2-8fcd-44fa-a838-b84ee5f44f0f
HUMAN PRESCRIPTION DRUG LABEL
Aug 4, 2023
EMD Serono, Inc.
DUNS: 088514898
Products 6
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
INTERFERON BETA-1A
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
INTERFERON BETA-1A
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INTERFERON BETA-1A
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
interferon beta-1a
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
INTERFERON BETA-1A
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
interferon beta-1a
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 12 Autoinjector Carton - 3344
NDC 44087-3344-1
Rebif® Rebidose®
(interferon beta-1a)
Injection
12 single-use autoinjectors
44 mcg / 0.5 mL
Rx only
For subcutaneous injection
Attention pharmacist: Each patient is required
to receive the enclosed Medication Guide
EMD
Serono
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: REBIF has not been tested for carcinogenic potential in animals.
Mutagenesis: Interferon beta was negative in an in vitro bacterial reverse mutation (Ames) assay and an in vitro cytogenetic assay in human lymphocytes in the presence and absence of metabolic activation.
Impairment of Fertility: In studies in normally cycling female cynomolgus monkeys given daily subcutaneous injections of interferon beta for six months at doses of up to 9 times the recommended weekly human dose (based on body surface area), no effects were observed on either menstrual cycling or serum estradiol levels. In male monkeys, the same doses of interferon beta had no demonstrable adverse effects on sperm count, motility, morphology, or function.
CLINICAL STUDIES SECTION
14 CLINICAL STUDIES
Two multicenter studies evaluated the safety and efficacy of REBIF in patients with relapsing-remitting multiple sclerosis.
Study 1 was a randomized, double-blind, placebo controlled study in patients with multiple sclerosis for at least one year, Kurtzke Expanded Disability Status Scale (EDSS) scores ranging from 0 to 5, and at least 2 acute exacerbations in the previous 2 years. Patients with chronic progressive forms of multiple sclerosis were excluded from the study. Patients received subcutaneous injections of either placebo (n = 187), REBIF 22 mcg (n = 189), or REBIF 44 mcg (n = 184) administered three times per week for two years. Doses of study agents were progressively increased to their target doses during the first 4 to 8 weeks for each patient in the study [see Dosage and Administration (2.1)].
The primary efficacy endpoint was the number of clinical exacerbations. Numerous secondary efficacy endpoints were also evaluated and included exacerbation-related parameters, effects of treatment on progression of disability and magnetic resonance imaging (MRI)-related parameters. Progression of disability was defined as an increase in the EDSS score of at least one point sustained for at least 3 months. Neurological examinations were completed every 3 months, during suspected exacerbations, and coincident with MRI scans. All patients underwent proton density T2-weighted (PD/T2) MRI scans at baseline and every 6 months. A subset of 198 patients underwent PD/T2 and T1-weighted gadolinium-enhanced (Gd)-MRI scans monthly for the first 9 months. Of the 560 patients enrolled, 533 (95%) provided 2 years of data and 502 (90%) received 2 years of study agent.
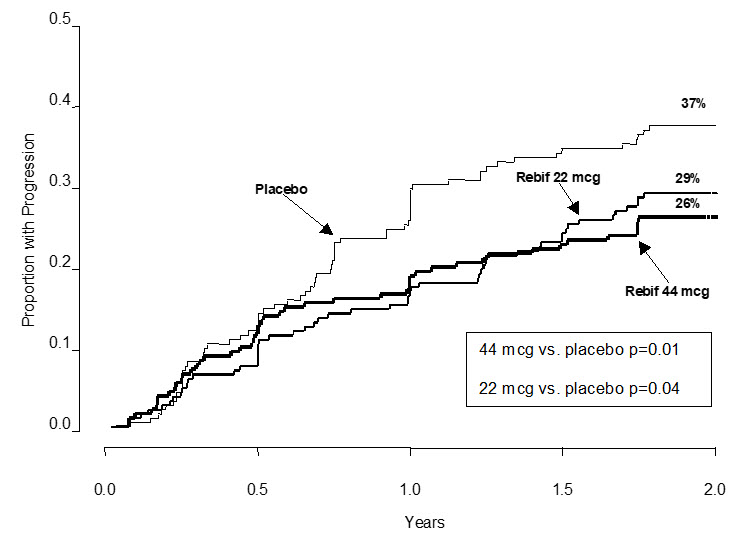
Study results are shown in Table 4 and Figure 1. REBIF at doses of 22 mcg and 44 mcg administered three times per week significantly reduced the number of exacerbations per patient as compared to placebo. Differences between the 22 mcg and 44 mcg groups were not significant (p >0.05).
Table 4: Clinical and MRI Endpoints from Study 1|
Placebo |
REBIF |
REBIF | |
|---|---|---|---|
|
n = 187 |
n = 189 |
n = 184 | |
Þ ß | |||
|
Exacerbation-related | |||
|
Mean number of exacerbations per patient over 2 years*,† |
2.56 |
1.82‡ |
1.73§ |
|
(Percent reduction) |
(29%) |
(32%) | |
|
Percent (%) of patients exacerbation-free at 2 years¶ |
15% |
25%# |
32%§ |
|
Median time to first exacerbation (months)*,Þ |
4.5 |
7.6‡ |
9.6§ |
|
MRI |
n = 172 |
n = 171 |
n = 171 |
|
Median percent (%) change of MRI PD-T2 lesion area at 2 yearsß |
11.0% |
-1.2%§ |
-3.8%§ |
|
Median number of active lesions per patient per scan (PD/T2; 6 monthly)ß |
2.25 |
0.75§ |
0.5§ |
The time to onset of progression in disability sustained for three months was significantly longer in patients treated with REBIF than in placebo-treated patients. The Kaplan-Meier estimates of the proportions of patients with sustained disability are depicted in Figure 1.
Figure 1: Proportions of Patients with Sustained Disability Progression
Study 2 was a randomized, open-label, evaluator-blinded, active comparator study. Patients with relapsing-remitting multiple sclerosis with EDSS scores ranging from 0 to 5.5, and at least 2 exacerbations in the previous 2 years were eligible for inclusion. Patients with chronic progressive forms of multiple sclerosis were excluded from the study. Patients were randomized to treatment with three times per week subcutaneous injections of REBIF 44 mcg (n=339) or once weekly intramuscular injections of 30 mcg AVONEX (n=338). Study duration was 48 weeks.
The primary efficacy endpoint was the proportion of patients who remained exacerbation-free at 24 weeks. The principal secondary endpoint was the mean number per patient per scan of combined unique active MRI lesions through 24 weeks, defined as any lesion that was T1 active or T2 active. Neurological examinations were performed every three months by a neurologist blinded to treatment assignment. Patient visits were conducted monthly, and mid-month telephone contacts were made to inquire about potential exacerbations. If an exacerbation was suspected, the patient was evaluated with a neurological examination. MRI scans were performed monthly and analyzed in a treatment- blinded manner.
Patients treated with REBIF 44 mcg three times per week were more likely to remain relapse-free at 24 and 48 weeks than were patients treated with AVONEX 30 mcg once per week (Table 5). This study does not support any conclusion regarding effects on the accumulation of physical disability.
Table 5: Clinical and MRI Results from Study 2|
REBIF |
AVONEX |
Absolute Difference |
Risk of relapse on REBIF relative to AVONEX | |
|---|---|---|---|---|
| ||||
|
Relapses |
N=339 |
N=338 | ||
|
Proportion of patients relapse-free at 24 weeks* |
75%† |
63% |
12% |
0.68 |
|
Proportion of patients relapse-free at 48 weeks |
62%‡ |
52% |
10% |
0.81 |
|
MRI (through 24 weeks) |
N=325 |
N=325 | ||
|
Median of the mean number of combined unique MRI lesions per patient per scan§ (25th, 75th percentiles) |
0.17† |
0.33 |
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
REBIF is supplied as a sterile solution containing no preservative available in the following package presentations:
Prefilled Syringes**:**
**REBIF (interferon beta -1a) Titration Pack, NDC 44087-8822-1**
- Six REBIF 8.8 mcg prefilled syringes and Six REBIF 22 mcg prefilled syringes
**REBIF (interferon beta -1a) 22 mcg Prefilled syringe**
- Twelve REBIF 22 mcg prefilled syringes, NDC 44087-0022**-**3
**REBIF (interferon beta -1a) 44 mcg Prefilled syringe**
- Twelve REBIF 44 mcg prefilled syringes, NDC 44087-0044-3
REBIF Rebidose Autoinjectors**:**
**REBIF Rebidose (interferon beta-1a) Titration Pack, NDC 44087-0188-1**
- Six REBIF Rebidose 8.8 mcg autoinjectors with lime-green injector buttons and Six REBIF Rebidose 22 mcg with yellow injector buttons. REBIF Rebidose (interferon beta-1a) 22 mcg Autoinjector
-
Twelve REBIF Rebidose 22 mcg autoinjectors with yellow injector buttons, NDC 44087-3322-1 REBIF Rebidose (interferon beta-1a) 44 mcg Autoinjector
-
Twelve REBIF Rebidose 44 mcg autoinjectors with teal-green injector buttons, NDC 44087-3344-1
REBIF should be stored refrigerated between 36°F to 46°F (2°C to 8°C). DO NOT FREEZE. If needed, REBIF may be stored between 36°F to 77°F (2°C to 25°C) for up to 30 days and away from heat and light, but refrigeration is preferred.
Do not use beyond the expiration date printed on packages. REBIF contains no preservatives. Each prefilled syringe and REBIF Rebidose autoinjector is intended for a single dose. Unused portions should be discarded.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Medication Guide).
Inform patients of the availability of a Medication Guide, and instruct them to read the Medication Guide prior to taking REBIF. Instruct patients to take REBIF only as prescribed.
Depression and Suicide
Advise patients that depression, suicidal ideation, and suicide have been reported during the use of REBIF. Inform patients of the symptoms of depression and suicidal ideation and instruct patients to immediately report any of these symptoms to their healthcare provider [see Warnings and Precautions (5.1)].
Hepatic Injury
Advise patients that severe liver injury, including hepatic failure, has been reported with the use of REBIF. Educate patients about the symptoms of hepatic injury and instruct patients to report them immediately to their healthcare provider [see Warnings and Precautions (5.2)].
Anaphylaxis and Other Allergic Reactions
Advise patients of the symptoms of allergic reactions and anaphylaxis, and instruct patients to seek immediate medical attention if these symptoms occur [see Warnings and Precautions (5.3)].
Injection Site Reactions Including Necrosis
Advise patients that injection site reactions occur in most patients treated with REBIF and that injection site necrosis may occur [see Warnings and Precautions (5.4)]. Instruct patients to promptly report any break in the skin, which may be associated with blue-black discoloration, swelling, or drainage of fluid from the injection site, prior to continuing their REBIF therapy.
To minimize the likelihood of injection site reactions, inform patients of the importance of rotating injection sites with each dose and the use of aseptic injection technique [see Dosage and Administration (2.2)]. Advise patients not to re-use needles or syringes and instruct patients on safe disposal procedures. Provide appropriate instruction for self-injection of REBIF and REBIF Rebidose, including careful review of the REBIF Medication Guide.
Decreased Peripheral Blood Counts
Advise patients that they may develop a lowering of their peripheral blood counts, including their white blood counts, red blood counts, and platelets, and that their blood counts will be checked during therapy with REBIF. Inform patients that they may be more likely to get infections, anemia, or be at risk for bleeding, and that they should contact their healthcare provider if they develop symptoms of these adverse reactions [see Warnings and Precautions (5.5)].
Pulmonary Arterial Hypertension
Inform patients that PAH has occurred in patients treated with interferon beta products, including REBIF. Instruct patients to promptly report any new symptoms such as new or increasing fatigue or shortness of breath to their healthcare provider [see Warnings and Precautions (5.7)].
Seizures
Instruct patients to report seizures immediately to their healthcare provider [see Warnings and Precautions (5.8)].
Flu-like Symptoms
Inform patients that flu-like symptoms are common following initiation of therapy with REBIF. Advise patients that concurrent use of analgesics and/or antipyretics may help reduce flu-like symptoms on treatment days [see Dosage and Administration (2.3)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant [see Use in Specific Populations (8.1)].
SPL MEDGUIDE SECTION
MEDICATION GUIDE
REBIF
(interferon beta-1a)
Injection for subcutaneous use
Read this Medication Guide before you start using REBIF and each time you get a refill. There may be new information. The information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about REBIF?
REBIF can cause serious side effects. Tell your healthcare provider right away if you have any of the symptoms listed below while taking REBIF.
**Behavioral health problems including depression and suicidal thoughts**. You may have mood problems including:
- depression (feeling hopeless or feeling bad about yourself)
- thoughts of hurting yourself or suicide
**Liver problems or worsening of liver problems including liver failure**.**Symptoms may include:**
|
|
|
During your treatment with REBIF you will need to see your healthcare provider regularly and have regular blood tests to check for side effects. |
**Serious allergic and skin reactions**.**Symptoms may include:**
- itching
- swelling of your face, eyes, lips, tongue or throat
- trouble breathing
- anxiousness
- feeling faint
- skin rash, hives, sores in your mouth, or skin blisters and peels
** Injection site problems.** REBIF may cause redness, pain, itching or swelling at the place where your injection was given. Call your healthcare provider right away if an injection site becomes swollen and painful or the area looks infected. You may have a skin infection or an area of severe skin damage (necrosis) requiring treatment by a healthcare provider.
What is REBIF?
REBIF is a prescription medicine used to treat relapsing forms of multiple sclerosis, to include clinically isolated syndrome, relapsing-remitting disease, and active secondary progressive disease, in adults. It is a form of protein called beta interferon that is produced in the body.
It is not known if REBIF is safe and effective in children.
Who should not take REBIF?
Do not take REBIF if you:
- are allergic to interferon beta, human albumin, or any of the ingredients in REBIF. See the end of this Medication Guide for a complete list of ingredients in REBIF.
What should I tell my healthcare provider before taking REBIF?
Before you take REBIF, tell your healthcare provider if you have or have had any of the following conditions:
- mental illness, including depression and suicidal behavior
- liver problems
- bleeding problems or blood clots
- low blood cell counts
- seizures (epilepsy)
- thyroid problems
- drink alcohol
- you are pregnant or plan to become pregnant. It is not known if REBIF can harm your unborn baby.
- you are breastfeeding or plan to breastfeed. REBIF may pass into your breastmilk. Talk with your healthcare provider about the best way to feed your baby if you take REBIF.
Tell your healthcare provider about all medicines you take, including prescription and over-the-counter medicines, vitamins and herbal supplements.
REBIF and other medicines may affect each other causing side effects.
Ask your healthcare provider or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I use REBIF?
*See the Instructions for Use at the end of this Medication Guide on how to prepare and give an injection of REBIF using a prefilled syringe. For the REBIF Rebidose autoinjector, read the Instructions for Use that comes with the REBIF Rebidose autoinjector.
- Your healthcare provider should show you how to prepare and measure your dose of REBIF and how to inject your REBIF before you use it for the first time.
- REBIF is given by injection under the skin (subcutaneous injection) on the same 3 days a week, for example, Monday, Wednesday and Friday.
- Your injections should be at least 48 hours apart. Take them the same time each day.
- Inject REBIF exactly as your healthcare provider tells you.
- Your healthcare provider will tell you how much REBIF to inject, and may change the dose based on how your body responds. Do not inject more than your healthcare provider tells you to.
- Do not change your dose unless your healthcare provider tells you to.
- Change (rotate) your injection site you choose with each injection. This will help decrease the chance that you will have an injection site reaction. *Do notinject REBIF into an area of the body where the skin is irritated, reddened, bruised, infected or scarred in any way.
- REBIF comes as a:
- prefilled syringe (REBIF)
- single-use prefilled autoinjector (REBIF Rebidose autoinjector) Your healthcare provider will decide which is best for you. Always use a new, unopened, prefilled syringe of REBIF or REBIF Rebidose autoinjector for each injection.Do notreuse prefilled syringes or REBIF Rebidose autoinjectors.
What are the possible side effects of REBIF?
REBIF may cause serious side effects, including:
*See "What is the most important information I should know about REBIF?" ***Blood problems.**REBIF can affect your bone marrow and cause low red and white blood cell, and platelet counts. In some people, these blood cell counts may fall to dangerously low levels. If your blood cell counts become very low, you can get infections and problems with bleeding and bruising. Your healthcare provider may ask you to have regular blood tests to check for blood problems. ***Pulmonary arterial hypertension.**Pulmonary arterial hypertension can occur with interferon beta products, including REBIF. Symptoms may include new fatigue or shortness of breath. Contact your healthcare provider right away if you develop these symptoms. ***Seizures.**Some people have had seizures while taking REBIF.
The most common side effects of REBIF include:
- flu-like symptoms. You may have flu-like symptoms when you first start taking REBIF. You may be able to manage these flu-like symptoms by taking over-the-counter pain and fever reducers. For many people, these symptoms lessen or go away over time. Symptoms may include:
- muscle aches
- fever
- tiredness
- chills
- stomach pain
- change in liver blood tests
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of REBIF. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store REBIF?
- Store REBIF in the refrigerator between 36°F to 46°F (2°C to 8°C). *Do notfreeze REBIF.
- If you cannot refrigerate your REBIF, you can store your REBIF at temperatures above 36°F and below 77°F (2°C to 25°C) for up to 30 days.
- Keep REBIF away from heat and light.
Keep REBIF and all medicines out of the reach of children.
General information about the safe and effective use of REBIF
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use REBIF for a condition for which it was not prescribed. Do not give REBIF to other people, even if they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about REBIF. If you would like more information, talk with your healthcare provider. You may ask your healthcare provider or pharmacist for information about REBIF that is written for healthcare professionals.
For more information, go to www.REBIF.com or call toll-free 1-877-447- 3243.
What are the ingredients in REBIF?
**Active ingredient:**interferon beta-1a
Inactive ingredients: albumin (human), mannitol, sodium acetate, water for injection
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Revised 7/2023
