Cisatracurium Besylate
These highlights do not include all the information needed to use CISATRACURIUM BESYLATE INJECTION safely and effectively. See full prescribing information for CISATRACURIUM BESYLATE INJECTION. CISATRACURIUM BESYLATE injection, for intravenous use Initial U.S. Approval: 1995
557d1d6b-9010-492f-b2d3-9558fbb56d01
HUMAN PRESCRIPTION DRUG LABEL
Sep 8, 2020
Sagent Pharmaceuticals
DUNS: 080579617
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
cisatracurium besylate
PRODUCT DETAILS
INGREDIENTS (2)
cisatracurium besylate
PRODUCT DETAILS
INGREDIENTS (2)
cisatracurium besylate
PRODUCT DETAILS
INGREDIENTS (3)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sticker Label
10 mg per mL
For Intravenous Use Only
For ICU Use Only
CISATRACURIUM BESYLATE INJECTION, USP

CONTRAINDICATIONS SECTION
4 CONTRAINDICATIONS
Cisatracurium besylate is contraindicated in patients with known hypersensitivity to cisatracurium. Severe anaphylactic reactions to cisatracurium besylate have been reported [see Warnings and Precautions (5.4)].
The use of 10 mL cisatracurium besylate multiple-dose vials is contraindicated for use in pediatric patients less than 1 month of age and low birth-weight infants because the formulation contains benzyl alcohol [see Warnings and Precautions (5.2) and Use in Specific Populations (8.4)].
- Known hypersensitivity to cisatracurium (4)
- 10 mL multiple-dose vials contain benzyl alcohol and are contraindicated in pediatric patients less than 1 month of age and low birth-weight infants (4)
ADVERSE REACTIONS SECTION
6 ADVERSE REACTIONS
6.1 Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Clinical Trials of Cisatracurium Besylate in Surgical Patients
The data presented below are based on studies involving 945 surgical patients who received cisatracurium besylate in conjunction with other drugs in US and European clinical studies in a variety of procedures [see Clinical Studies (14.1)].
Table 3 displays adverse reactions that occurred at a rate of less than 1%.
Table 3. Adverse Reactions in Clinical Trials of Cisatracurium Besylate in Surgical Patients|
Adverse Reaction |
Incidence |
|
Bradycardia |
0.4% |
|
Hypotension |
0.2% |
|
Flushing |
0.2% |
|
Bronchospasm |
0.2% |
|
Rash |
0.1% |
Adverse Reactions in Clinical Trials of Cisatracurium Besylate in Intensive Care Unit Patients
The adverse reactions presented below were from studies involving 68 adult ICU patients who received cisatracurium besylate in conjunction with other drugs in US and European clinical studies [see Clinical Studies (14.3)]. One patient experienced bronchospasm. In one of the two ICU studies, a randomized and double-blind study of ICU patients using TOF neuromuscular monitoring, there were two reports of prolonged recovery (range: 167 and 270 minutes) among 28 patients administered cisatracurium besylate and 13 reports of prolonged recovery (range: 90 minutes to 33 hours) among 30 patients administered vecuronium.
6.2 Postmarketing Experience
The following events have been identified during post-approval use of cisatracurium besylate in conjunction with one or more anesthetic agents in clinical practice. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potential causal connection to cisatracurium besylate: anaphylaxis, histamine release, prolonged neuromuscular block, muscle weakness, myopathy.
The most common adverse reactions (0.1% to 0.4%) were bradycardia, hypotension, flushing, bronchospasm, and rash. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Sagent Pharmaceuticals, Inc. at 1-866-625-1618 or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DRUG INTERACTIONS SECTION
7 DRUG INTERACTIONS
7.1 Clinically Significant Drug Interactions
Table 4 displays clinically significant drug interactions with cisatracurium besylate.
Table 4. Clinically Significant Drug Interactions with Cisatracurium Besylate
| |
|
† Examples: aminoglycosides, tetracyclines, bacitracin, polymyxins, lincomycin, clindamycin, colistin, sodium colistimethate | |
|
Drug or Drug Class |
Clinical Implications***** |
|
Succinylcholine |
The use of succinylcholine prior to cisatracurium besylate administration may decrease the time to onset of maximum neuromuscular blockade but has no effect on the duration of neuromuscular blockade. |
|
Inhalational Anesthetics |
Administration of inhalational anesthetics with nitrous oxide/oxygen for greater than 30 minutes to achieve 1.25 Minimum Alveolar Concentration (MAC) may prolong the duration of action of initial and maintenance doses of cisatracurium besylate. This may potentiate the neuromuscular blockade. |
|
Antibiotics† |
May prolong the neuromuscular blockade action of cisatracurium besylate |
|
Phenytoin, Carbamazepine |
May increase resistance to the neuromuscular blockade action of cisatracurium besylate resulting in shorter durations of neuromuscular blockade and infusion rate requirements may be higher. |
7.2 Drugs Without Clinically Significant Drug Interactions With
Cisatracurium Besylate
In clinical studies, propofol had no effect on the duration of action or dosing requirements for cisatracurium besylate. Cisatracurium besylate is not compatible with propofol for Y-site administration.
- Succinylcholine: May decrease time to onset of maximum neuromuscular blockade (7.1)
- Inhalational anesthetics, antibiotics, local anesthetics, magnesium salts, procainamide, lithium, quinidine: May potentiate or prolong neuromuscular blockade action of cisatracurium besylate. Use peripheral nerve stimulator and monitor clinical signs of neuromuscular blockade. (5.8, 7.1)
- Phenytoin and Carbamazepine: May shorten duration of neuromuscular blockade. Use peripheral nerve stimulator and monitor clinical signs of neuromuscular blockade. (5.9, 7.1)
DESCRIPTION SECTION
11 DESCRIPTION
Cisatracurium Besylate Injection, USP is a nondepolarizing skeletal neuromuscular blocker for intravenous administration. Compared to other neuromuscular blockers, it is intermediate in its onset and duration of action. Cisatracurium besylate is one of 10 isomers of atracurium besylate and constitutes approximately 15% of that mixture. Cisatracurium besylate is [1R- [1α,2α(1'R*,2'R*)]]-2,2'-[1,5-pentanediylbis[oxy(3-oxo-3,1-propanediyl)]]bis[1-[(3,4-dimethoxyphenyl)methyl]-1,2,3,4-tetrahydro-6,7-dimethoxy-2-methylisoquinolinium] dibenzenesulfonate. The molecular formula of the cisatracurium parent bis- cation is C53H72N2O12 and the molecular weight is 929.2. The molecular formula of cisatracurium as the besylate salt is C65H82N2O18S2 and the molecular weight is 1243.50. The structural formula of cisatracurium besylate is:
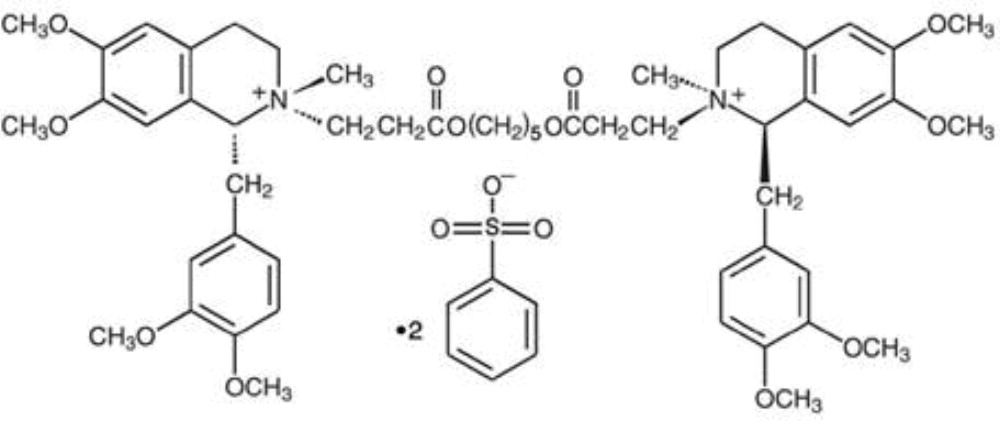
The log of the partition coefficient of cisatracurium besylate is -2.12 in a 1-octanol/distilled water system at 25°C.
Cisatracurium Besylate Injection, USP is a sterile, non-pyrogenic aqueous solution provided in 5 mL, 10 mL, and 20 mL vials. The pH is adjusted to 3.25 to 3.65 with benzenesulfonic acid.
- The 5 mL single-dose vials contain 2 mg per mL cisatracurium, equivalent to 2.68 mg per mL cisatracurium besylate.
- The 10 mL multiple-dose vials contain 2 mg per mL cisatracurium, equivalent to 2.68 mg per mL cisatracurium besylate, and 0.9% benzyl alcohol as a preservative.
- The 20 mL single-dose vials contain 10 mg per mL cisatracurium, equivalent to 13.38 mg per mL cisatracurium besylate.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term animal studies to evaluate the carcinogenic potential of cisatracurium besylate have not been performed.
Mutagenesis
Cisatracurium besylate was evaluated in a battery of four genotoxicity assays. Evaluation of cisatracurium besylate in the in vitro mouse lymphoma forward gene mutation assay resulted in mutations in the presence and absence of exogenous metabolic activation. The in vitro bacterial reverse gene mutation (Ames) assay, in vitro human lymphocyte chromosomal aberration assay, and an in vivo rat bone marrow cytogenetic assay did not demonstrate evidence of mutagenicity or clastogenicity.
Impairment of Fertility
Studies to determine if cisatracurium besylate impacts fertility have not been completed.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
Cisatracurium Besylate Injection, USP is a clear solution supplied as follows:
|
NDC |
Cisatracurium Besylate Injection, USP |
Package Factor |
|
25021-670-10 |
20 mg per 10 mL Multi-Dose Vial |
10 vials per carton |
|
25021-668-05 |
10 mg per 5 mL Single-Dose Vial |
10 vials per carton |
NOTE: Multiple-Dose Vial contains 0.9% w/v benzyl alcohol as a preservative [see Warnings and Precautions (5.2)].
|
NDC |
Cisatracurium Besylate Injection, USP |
Package Factor |
|
25021-669-20 |
200 mg per 20 mL Single-Dose Vial* |
1 vial per carton |
*****Intended only for use in the ICU.
Storage
Refrigerate Cisatracurium Besylate Injection, USP at 2° to 8°C (36° to 46°F) in the carton to preserve potency. Upon removal from refrigeration to room temperature storage conditions (25°C/77°F), use Cisatracurium Besylate Injection, USP within 21 days even if re-refrigerated.
Do not freeze.
Protect from light. Retain in carton until time of use.
Single-Dose Vials: Preservative-free. Discard unused portion.
Sterile, Nonpyrogenic.
The container closure is not made with natural rubber latex.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Hypersensitivity Reactions Including Anaphylaxis
Advise the caregiver and/or family that severe hypersensitivity reactions have occurred with cisatracurium besylate [see Warnings and Precautions (5.4)].
SAGENT®
Mfd. for SAGENT Pharmaceuticals
Schaumburg, IL 60195 (USA)
Made in India
©2020 Sagent Pharmaceuticals, Inc.
September 2020
SAGENT Pharmaceuticals®
DOSAGE & ADMINISTRATION SECTION
2 DOSAGE AND ADMINISTRATION
2.1 Important Dosage and Administration Instructions
Important administration instructions include:
- Cisatracurium besylate injection is for intravenous use only.
- Administer cisatracurium besylate injection in carefully adjusted dosage by or under the supervision of experienced clinicians who are familiar with the drug's actions and the possible complications.
- Use cisatracurium besylate injection only if the following are immediately available: personnel and facilities for resuscitation and life support (tracheal intubation, artificial ventilation, oxygen therapy); and an antagonist of cisatracurium besylate injection [see Overdosage (10)].
- The dosage information which follows is intended to serve as an initial guide for individual patients; base subsequent cisatracurium besylate injection dosage on the patients' responses to the initial doses.
- Use a peripheral nerve stimulator to:
- Determine the adequacy of neuromuscular blockade (e.g., need for additional cisatracurium besylate injection doses, reduction of the infusion rate).
- Minimize risk of overdosage or underdosage.
- Assess the extent of recovery from neuromuscular blockade (e.g., spontaneous recovery or recovery after administration of a reversal agent, e.g., neostigmine).
- Appropriately titrate doses to potentially limit exposure to toxic metabolites.
- Facilitate more rapid reversal of the cisatracurium besylate injection-induced paralysis.
Risk of Medication Errors
Accidental administration of neuromuscular blocking agents may be fatal. Store cisatracurium besylate injection with the cap and ferrule intact and in a manner that minimizes the possibility of selecting the wrong product [see Warnings and Precautions (5.5)].
2.2 Recommended Cisatracurium Besylate Injection Dose for Performing
Tracheal Intubation
Tracheal Intubation in Adults
Prior to selecting the initial cisatracurium besylate injection bolus dose, consider the desired time to tracheal intubation and the anticipated length of surgery, factors affecting time to onset of complete neuromuscular block such as age and renal function, and factors that may influence intubation conditions such as the presence of co-induction agents (e.g., fentanyl and midazolam) and the depth of anesthesia.
In conjunction with a propofol/nitrous oxide/oxygen induction-intubation technique or a thiopental/nitrous oxide/oxygen induction-intubation technique, the recommended starting weight-based dose of cisatracurium besylate injection is between 0.15 mg/kg and 0.2 mg/kg administered by bolus intravenous injection. Doses up to 0.4 mg/kg have been safely administered by bolus intravenous injection to healthy patients and patients with serious cardiovascular disease [see Clinical Pharmacology (12.2)].
Patients with Neuromuscular Disease
The maximum recommended initial bolus dose of cisatracurium besylate injection is 0.02 mg/kg in patients with neuromuscular diseases (e.g., myasthenia gravis and myasthenic syndrome and carcinomatosis) [see Warnings and Precautions (5.1)].
Geriatric Patients and Patients with End-Stage Renal Disease
Because the time to maximum neuromuscular blockade is approximately 1 minute slower in geriatric patients compared to younger patients (and in patients with end-stage renal disease than in patients with normal renal function), consider extending the interval between administering cisatracurium besylate injection and attempting intubation by at least 1 minute to achieve adequate intubation conditions in geriatric patients and patients with end-stage renal disease. A peripheral nerve stimulator should be used to determine the adequacy of muscle relaxation for the purposes of intubation and the timing and amounts of subsequent doses [see Use in Specific Populations (8.5, 8.6) and Clinical Pharmacology (12.3)].
Tracheal Intubation in Pediatric Patients
Infants 1 to 23 Months of Age
The recommended dose of cisatracurium besylate injection for intubation of pediatric patients ages 1 month to 23 months is 0.15 mg/kg administered over 5 to 10 seconds. When administered during stable opioid/nitrous oxide/oxygen anesthesia, 0.15 mg/kg of cisatracurium besylate injection produced maximum neuromuscular blockade in about 2 minutes (range: 1.3 to 4.3 minutes) with a clinically effective block (time to 25% recovery) for about 43 minutes (range: 34 to 58 minutes) [see Clinical Studies (14.2)].
Pediatric Patients 2 to 12 Years of Age
The recommended weight-based bolus dose of cisatracurium besylate injection for pediatric patients 2 to 12 years of age is 0.1 to 0.15 mg/kg administered over 5 to 10 seconds. When administered during stable opioid/nitrous oxide/oxygen anesthesia, 0.1 mg/kg cisatracurium besylate injection produced maximum neuromuscular blockade in an average of 2.8 minutes (range: 1.8 to 6.7 minutes) with a clinically effective block (time to 25% recovery) for 28 minutes (range: 21 to 38 minutes). When administered during stable opioid/nitrous oxide/oxygen anesthesia, 0.15 mg/kg cisatracurium besylate injection produced maximum neuromuscular blockade in an average of about 3 minutes (range: 1.5 to 8 minutes) with a clinically effective block for 36 minutes (range: 29 to 46 minutes) [see Clinical Studies (14.2)].
2.3 Recommended Maintenance Bolus Cisatracurium Besylate Injection Doses in
Adult Surgical Procedures
Determine if maintenance bolus doses are needed based on clinical criteria including the response to peripheral nerve stimulation. The recommended maintenance bolus dose of cisatracurium besylate injection is 0.03 mg/kg; however, smaller or larger maintenance doses may be administered based on the required duration of action. Administer the first maintenance bolus dose starting:
- 40 to 50 minutes after an initial dose of cisatracurium besylate injection 0.15 mg/kg;
- 50 to 60 minutes after an initial dose of cisatracurium besylate injection 0.2 mg/kg.
For long surgical procedures using inhalational anesthetics administered with nitrous oxide/oxygen at the 1.25 MAC level for at least 30 minutes, consider administering less frequent maintenance bolus doses or lower maintenance bolus doses of cisatracurium besylate injection [see Clinical Pharmacology (12.2)]. No adjustment to the initial cisatracurium besylate injection maintenance bolus dose should be necessary when cisatracurium besylate injection is administered shortly after initiation of volatile agents or when used in patients receiving propofol anesthesia.
2.4 Dosage in Burn Patients
Burn patients have been shown to develop resistance to nondepolarizing neuromuscular blocking agents; therefore, consider increasing the cisatracurium besylate injection dosages for intubation and maintenance [see Use in Specific Populations (8.8)].
2.5 Dosage for Continuous Infusion
Continuous Infusion for Surgical Procedures in Adults and Pediatric Patients
During extended surgical procedures, cisatracurium besylate injection may be administered by continuous infusion to adults and pediatric patients aged 2 or more years if patients have spontaneous recovery after the initial cisatracurium besylate injection bolus dose. Following recovery from neuromuscular blockade, it may be necessary to re-administer a bolus dose to quickly re-establish neuromuscular blockade prior to starting the continuous infusion.
If patients have had recovery of neuromuscular function, the recommended initial cisatracurium besylate injection infusion rate is 3 mcg/kg/minute [see Dosage and Administration (2.6)]. Subsequently reduce the rate to 1 to 2 mcg/kg/minute to maintain continuous neuromuscular blockade. Use peripheral nerve stimulation to assess the level of neuromuscular blockade and to appropriately titrate the cisatracurium besylate injection infusion rate. If no response is elicited to peripheral nerve stimulation, discontinue the infusion until a response returns.
Consider reducing the infusion rate by up to 30% to 40% when cisatracurium besylate injection is administered during stable isoflurane anesthesia for at least 30 minutes (administered with nitrous oxide/oxygen at the 1.25 MAC level) [see Clinical Pharmacology (12.2)]. Greater reductions in the cisatracurium besylate injection infusion rate may be required with longer durations of administration of isoflurane or with the administration of other inhalational anesthetics.
Patients Undergoing Coronary Artery Bypass Graft (CABG) Surgery
Consider reducing the infusion rate in patients undergoing CABG with induced hypothermia to half the rate required during normothermia [see Clinical Pharmacology (12.2)]. Spontaneous recovery from neuromuscular block following discontinuation of infusion of cisatracurium besylate injection infusion is expected to proceed at a rate comparable to that following administration of a single bolus dose.
Continuous Infusion for Mechanical Ventilation in the Intensive Care Unit in Adults
During extended need for mechanical ventilation and skeletal muscle relaxation in the intensive care unit (ICU), cisatracurium besylate injection may be administered by continuous infusion to adults if a patient has spontaneous recovery of neuromuscular function after the initial cisatracurium besylate injection bolus dose. Following recovery from neuromuscular blockade, it may be necessary to re-administer a bolus dose to quickly re-establish neuromuscular blockade prior to starting the continuous infusion.
The recommended cisatracurium besylate injection infusion rate in adult patients in the ICU is 3 mcg/kg/minute (range: 0.5 to 10.2 mcg/kg/minute) [see Dosage and Administration (2.6)]. Use peripheral nerve stimulation to assess the level of neuromuscular blockade and to appropriately titrate the cisatracurium besylate injection infusion rate.
2.6 Rate Tables for Continuous Infusion
The intravenous infusion rate depends upon the cisatracurium besylate injection concentration, the desired dose, the patient's weight, and the contribution of the infusion solution to the fluid requirements of the patient. Tables 1 and 2 provide guidelines for the cisatracurium besylate injection infusion rate, in mL/hour (equivalent to microdrops/minute when 60 microdrops = 1 mL), in concentrations of 0.1 mg per mL or 0.4 mg per mL, respectively.
Table 1. Cisatracurium Besylate Injection Infusion Rates for Maintenance of Neuromuscular Blockade During Opioid/Nitrous Oxide/Oxygen Anesthesia with a Concentration of 0.1 mg per mL|
Drug Delivery Rate (mcg/kg/minute) | |||||
|
1 |
1.5 |
2 |
3 |
5 | |
|
Patient Weight |
Infusion Delivery Rate (mL/hour) | ||||
|
10 kg |
6 |
9 |
12 |
18 |
30 |
|
45 kg |
27 |
41 |
54 |
81 |
135 |
|
70 kg |
42 |
63 |
84 |
126 |
210 |
|
100 kg |
60 |
90 |
120 |
180 |
300 |
|
Drug Delivery Rate (mcg/kg/minute) | |||||
|
1 |
1.5 |
2 |
3 |
5 | |
|
Patient Weight |
Infusion Delivery Rate (mL/hour) | ||||
|
10 kg |
1.5 |
2.3 |
3 |
4.5 |
7.5 |
|
45 kg |
6.8 |
10.1 |
13.5 |
20.3 |
33.8 |
|
70 kg |
10.5 |
15.8 |
21 |
31.5 |
52.5 |
|
100 kg |
15 |
22.5 |
30 |
45 |
75 |
2.7 Preparation of Cisatracurium Besylate Injection
Visually inspect cisatracurium besylate injection for particulate matter and discoloration prior to administration. If a cisatracurium besylate injection solution is cloudy or contains visible particulates, do not use cisatracurium besylate injection. Cisatracurium besylate injection is a colorless to slightly yellow or greenish-yellow solution.
Cisatracurium besylate injection may be diluted to 0.1 mg per mL in the following solutions:
- 5% Dextrose Injection, USP
- 0.9% Sodium Chloride Injection, USP, or
- 5% Dextrose and 0.9% Sodium Chloride Injection, USP
Store these diluted cisatracurium besylate injection solutions either in a refrigerator or at room temperature for 24 hours without significant loss of potency.
Cisatracurium besylate injection also may be diluted to 0.1 mg per mL or 0.2 mg per mL in the following solution:
- Lactated Ringer's and 5% Dextrose Injection
Store this diluted cisatracurium besylate injection solution under refrigeration for no more than 24 hours.
Do not dilute cisatracurium besylate injection in Lactated Ringer's Injection, USP due to chemical instability.
2.8 Drug Compatibility
Cisatracurium besylate injection is compatible and may be administered with the following solutions through Y-site administration:
- 5% Dextrose Injection, USP
- 0.9% Sodium Chloride Injection, USP
- 5% Dextrose and 0.9% Sodium Chloride Injection, USP
- Sufentanil Citrate Injection, diluted as directed
- Alfentanil Hydrochloride Injection, diluted as directed
- Fentanyl Citrate Injection, diluted as directed
- Midazolam Hydrochloride Injection, diluted as directed
- Droperidol Injection, diluted as directed
Cisatracurium besylate injection is acidic (pH = 3.25 to 3.65) and may not be compatible with alkaline solution having a pH greater than 8.5 (e.g., barbiturate solutions). Therefore, do not administer cisatracurium besylate injection and alkaline solutions simultaneously in the same intravenous line.
Cisatracurium besylate injection is not compatible with propofol injection or ketorolac injection for Y-site administration. Compatibility studies with other parenteral products have not been conducted.
- Administer intravenously only by or under the supervision of experienced clinicians familiar with drug's actions and possible complications (2.1)
- Use only if personnel and facilities for resuscitation and life support, and a cisatracurium besylate injection antagonist are immediately available (2.1)
- Use a peripheral nerve stimulator to determine adequacy of blockade (e.g., need for additional doses), minimize risk of overdosage or underdosage, assess extent of recovery from blockade, potentially limit exposure to toxic metabolites through dose titration, and facilitate more rapid reversal of cisatracurium besylate injection-induced paralysis (2.1)
- See the Full Prescribing Information for:
- Dosage and administration instructions in adults, pediatric patients, geriatric patients, patients with neuromuscular disease, burns, end-stage renal disease, and patients undergoing coronary artery bypass graft surgery with induced hypothermia (2.2, 2.3, 2.4, 2.5)
- Continuous infusion rates (2.6)
- Preparation instructions (2.7)
- Drug compatibility (2.8)
