SOUTH MOON Ingrown Toenail Drops
3f10b998-49c7-f2b0-e063-6294a90ac7bf
HUMAN OTC DRUG LABEL
Sep 18, 2025
Shantou South Moon Biotechnology Co., Ltd.
DUNS: 457126192
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
GLYCERIN
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (6)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
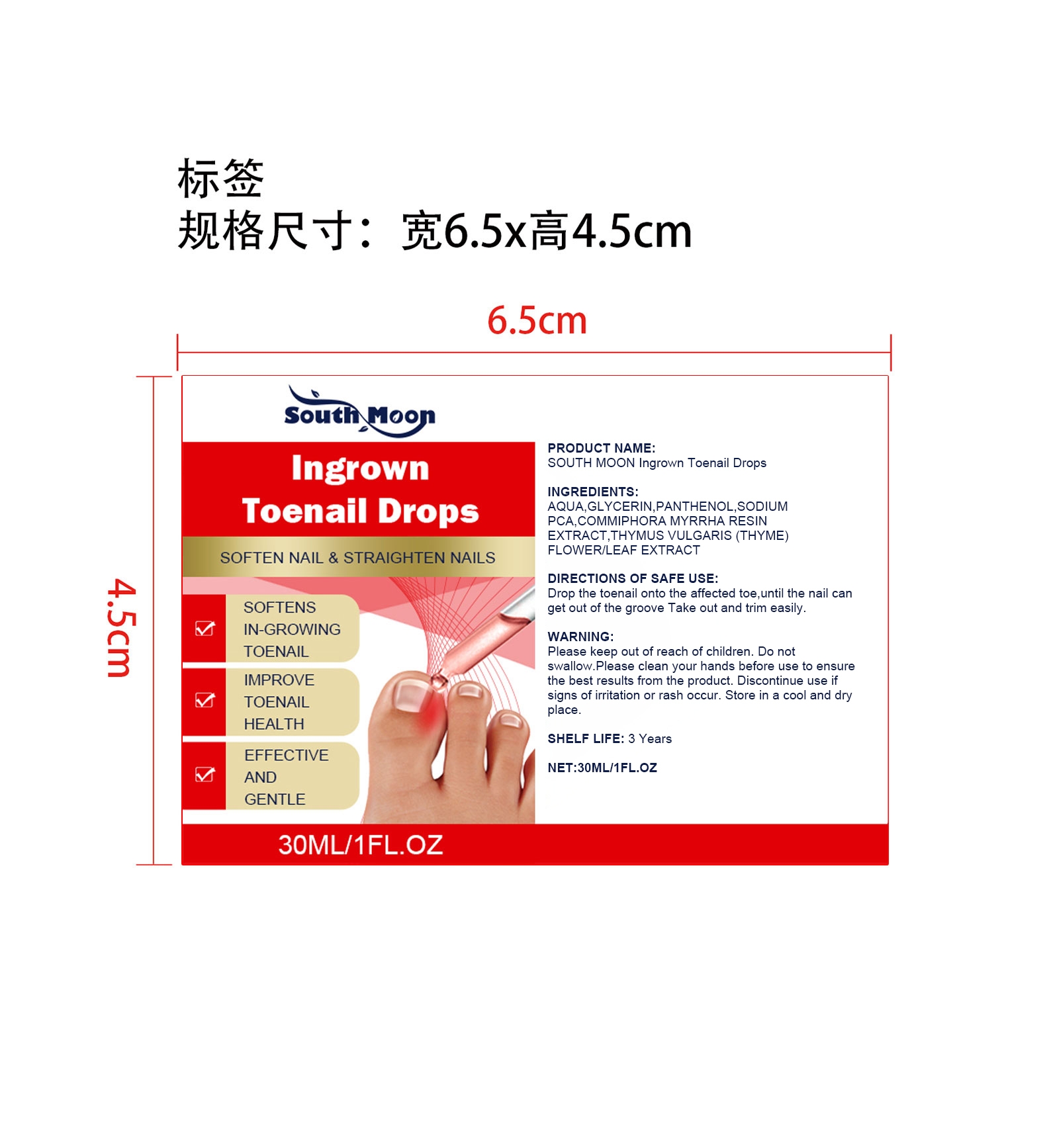
INDICATIONS & USAGE SECTION
Drop the toenail onto the affected toe,until the nail can get out of the groove Take out and trim easily.
DOSAGE & ADMINISTRATION SECTION
Drop the toenail onto the affected toe,until the nail can get out of the groove Take out and trim easily.
STORAGE AND HANDLING SECTION
Store in a cool and dry place.
INACTIVE INGREDIENT SECTION
AQUA、GLYCERIN、PANTHENOL、SODIUM PCA
OTC - ACTIVE INGREDIENT SECTION
COMMIPHORA MYRRHA RESIN、THYMUS VULGARIS (THYME) FLOWER/LEAF OIL
OTC - WHEN USING SECTION
Drop the toenail onto the affected toe,until the nail can get out of the groove Take out and trim easily.
OTC - PURPOSE SECTION
SOFTENS IN-GROWING TOENAIL
IMPROVE TOENAIL HEALTH
EFFECTIVE AND GENTLE
WARNINGS SECTION
Please keep out of reach of children. Do not swallow.Please clean your hands before use to ensure the best results from the product. Discontinue use if signs of irritation or rash occur. Store in a cool and dry place.
OTC - DO NOT USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - STOP USE SECTION
Discontinue use if signs of irritation or rash occur.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Please keep out of reach of children. Do not swallow.
