Antigingivitis/Antiplaque
Meijer D00.001/D00AA Alcohol-Free Gum Defense Mouthwash
ea55487c-a13f-41bd-b952-a7d848480ffa
HUMAN OTC DRUG LABEL
Aug 12, 2025
Meijer Distribution, INC
DUNS: 006959555
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Cetylpyridinium Chloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (11)
Drug Labeling Information
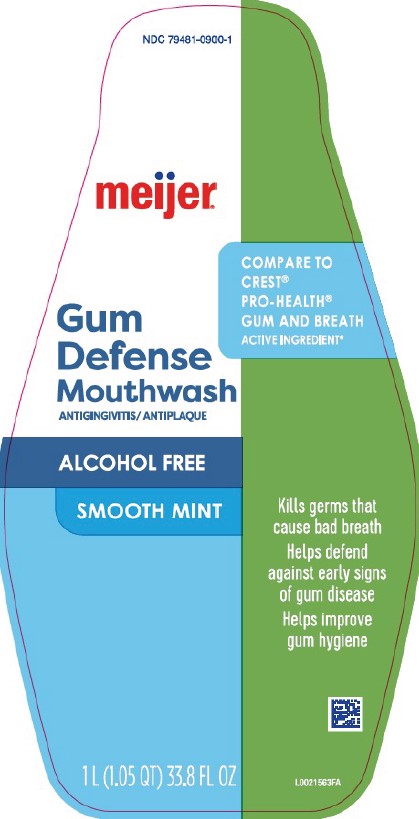
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Principal Display Panel
NDC 79481-0900-1
meijer ®
COMPARE TO CREST ®PRO-HEALTH ® GUM AND BREATH ACTIVE INGREDIENT*
Gum Defense Mouthwash
ANTIGINGIVITIS/ANTIPLAQUE
ALCOHOL FREE
SMOOTH MINT
Kills germs that cause bad breath
Helps defend against early signs of gum disease
Helps improve gum hygiene
1 L (1.5 QT) 33.8 FL OZ
INDICATIONS & USAGE SECTION
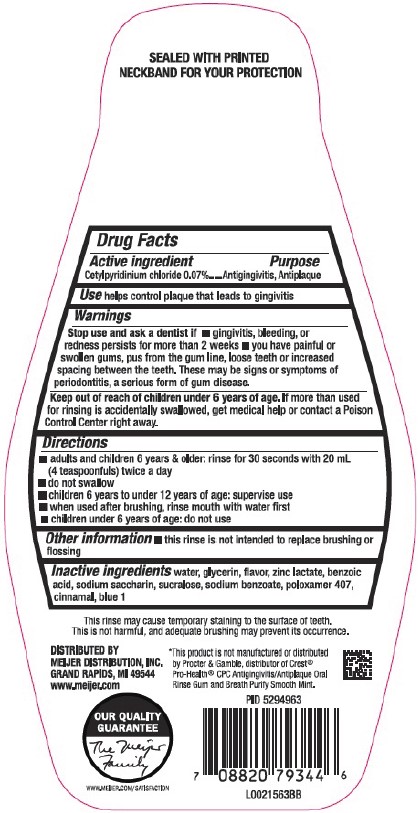
Use
helps control plaque that leads to gingivitis
ADVERSE REACTIONS SECTION
ADVERSE REACTION
DISTRIBUTED BY
MEIJER DISTRIBUTION, INC.
GRAND RAPIDS, MI 49544
www.meijer.com
OUR QUALITY GUARANTEE
The Meijer Family
WWW.MEIJER.COM/SATISFACTION
WARNINGS SECTION
Warnings
For this product
OTC - ACTIVE INGREDIENT SECTION
Active Ingredient
Cetylpyridinium Chloride 0.07%
OTHER SAFETY INFORMATION
Tamper Evident Statement
SEALED WITH PRINTED NECKBAND FOR YOUR PROTECTION
OTC - PURPOSE SECTION
Purpose
Antigingivitis, Antiplaque
OTC - STOP USE SECTION
Stop use and ask a dentist if
- gingivitis, bleeding, or redness persists for more than 2 weeks
- you have painful or swollen gums, pus from the gum line, loose teeth or increased spacing between the teeth. These may be signs or symptoms of periodontitis, a serious form of gum disease.
OTC - KEEP OUT OF REACH OF CHILDREN SECTION
Keep out of reach of children under 6 years of age.
If more than used for rinsing is accidentally swallowed, get medical help or contact a Poison Control Center right away.
DOSAGE & ADMINISTRATION SECTION
Directions
- adults and children 6 years & older: rinse for 30 seconds with 20 mL (4 teaspoonfuls) twice a day
- do not swallow
- children 6 years to under 12 years of age: supervise use
- when used after brushing, rinse mouth with water first
- children under 6 years of age: do not use
SPL UNCLASSIFIED SECTION
Disclaimers
This may cause temporary staining to the surface of teeth. This is not harmful, and adequate brushing may prevent its occurrence.
*This product is not manufactured or distributed by Procter & Gamble, distributor of Crest ® Pro-Health ® CPC Antiginigivitis/Antiplaque Oral Rinse Gum and Breath Purify Smooth Mint.
INACTIVE INGREDIENT SECTION
Inactive ingredients
water, glycerin, flavor, zinc lactate, benzoic acid, sodium saccharin, sucralose, sodium benzoate, poloxamer 407, cinnamal, blue 1
