Durela
Approved
Approval ID
d787c214-e8fe-1da1-e053-2a95a90a4288
Product Type
HUMAN PRESCRIPTION DRUG LABEL
Effective Date
Dec 19, 2022
Manufacturers
FDA
Galephar Pharmaceutical Research Inc.
DUNS: 003551624
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Tramadol Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code66277-239
Product Classification
G
Generic Name
Tramadol Hydrochloride
Product Specifications
Route of AdministrationORAL
Effective DateDecember 19, 2022
FDA Product Classification
INGREDIENTS (11)
POVIDONE K30Inactive
Code: U725QWY32X
Classification: IACT
LACTOSE MONOHYDRATEInactive
Code: EWQ57Q8I5X
Classification: IACT
POLYSORBATE 80Inactive
Code: 6OZP39ZG8H
Classification: IACT
CELLULOSE, MICROCRYSTALLINEInactive
Code: OP1R32D61U
Classification: IACT
HYPROMELLOSE 2910 (6 MPA.S)Inactive
Code: 0WZ8WG20P6
Classification: IACT
SUCROSE STEARATEInactive
Code: 274KW0O50M
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
TALCInactive
Code: 7SEV7J4R1U
Classification: IACT
STARCH, CORNInactive
Code: O8232NY3SJ
Classification: IACT
ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW)Inactive
Code: P2OM2Q86BI
Classification: IACT
TRAMADOL HYDROCHLORIDEActive
Quantity: 100 mg in 1 1
Code: 9N7R477WCK
Classification: ACTIB
Tramadol Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code66277-242
Product Classification
G
Generic Name
Tramadol Hydrochloride
Product Specifications
Route of AdministrationORAL
Effective DateDecember 19, 2022
FDA Product Classification
INGREDIENTS (11)
POVIDONE K30Inactive
Code: U725QWY32X
Classification: IACT
LACTOSE MONOHYDRATEInactive
Code: EWQ57Q8I5X
Classification: IACT
CELLULOSE, MICROCRYSTALLINEInactive
Code: OP1R32D61U
Classification: IACT
POLYSORBATE 80Inactive
Code: 6OZP39ZG8H
Classification: IACT
ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW)Inactive
Code: P2OM2Q86BI
Classification: IACT
SUCROSE STEARATEInactive
Code: 274KW0O50M
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
TALCInactive
Code: 7SEV7J4R1U
Classification: IACT
TRAMADOL HYDROCHLORIDEActive
Quantity: 300 mg in 1 1
Code: 9N7R477WCK
Classification: ACTIB
STARCH, CORNInactive
Code: O8232NY3SJ
Classification: IACT
HYPROMELLOSE 2910 (6 MPA.S)Inactive
Code: 0WZ8WG20P6
Classification: IACT
Tramadol Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
NDC Product Code66277-241
Product Classification
G
Generic Name
Tramadol Hydrochloride
Product Specifications
Route of AdministrationORAL
Effective DateDecember 19, 2022
FDA Product Classification
INGREDIENTS (11)
LACTOSE MONOHYDRATEInactive
Code: EWQ57Q8I5X
Classification: IACT
POVIDONE K30Inactive
Code: U725QWY32X
Classification: IACT
ETHYL ACRYLATE AND METHYL METHACRYLATE COPOLYMER (2:1; 750000 MW)Inactive
Code: P2OM2Q86BI
Classification: IACT
CELLULOSE, MICROCRYSTALLINEInactive
Code: OP1R32D61U
Classification: IACT
POLYSORBATE 80Inactive
Code: 6OZP39ZG8H
Classification: IACT
MAGNESIUM STEARATEInactive
Code: 70097M6I30
Classification: IACT
HYPROMELLOSE 2910 (6 MPA.S)Inactive
Code: 0WZ8WG20P6
Classification: IACT
TALCInactive
Code: 7SEV7J4R1U
Classification: IACT
TRAMADOL HYDROCHLORIDEActive
Quantity: 200 mg in 1 1
Code: 9N7R477WCK
Classification: ACTIB
STARCH, CORNInactive
Code: O8232NY3SJ
Classification: IACT
SUCROSE STEARATEInactive
Code: 274KW0O50M
Classification: IACT
Drug Labeling Information
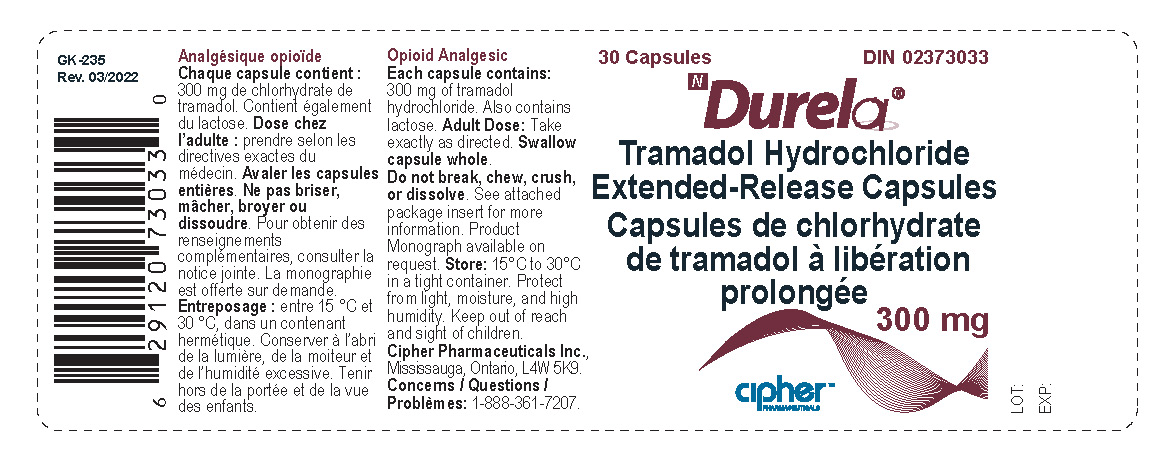
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
LOINC: 51945-4Updated: 5/16/2022
Durela 300 mg