Neutrogena Boost Your Skins Vitals Team Regenerate
Neutrogena Boost Your Skins Vitals Team Regenerate
0acd9110-4ba5-227a-e063-6294a90ad942
HUMAN OTC DRUG LABEL
Jul 1, 2025
Kenvue Brands LLC
DUNS: 118772437
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Avobenzone, Homosalate, Octisalate, and Octocrylene
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
Drug Labeling Information
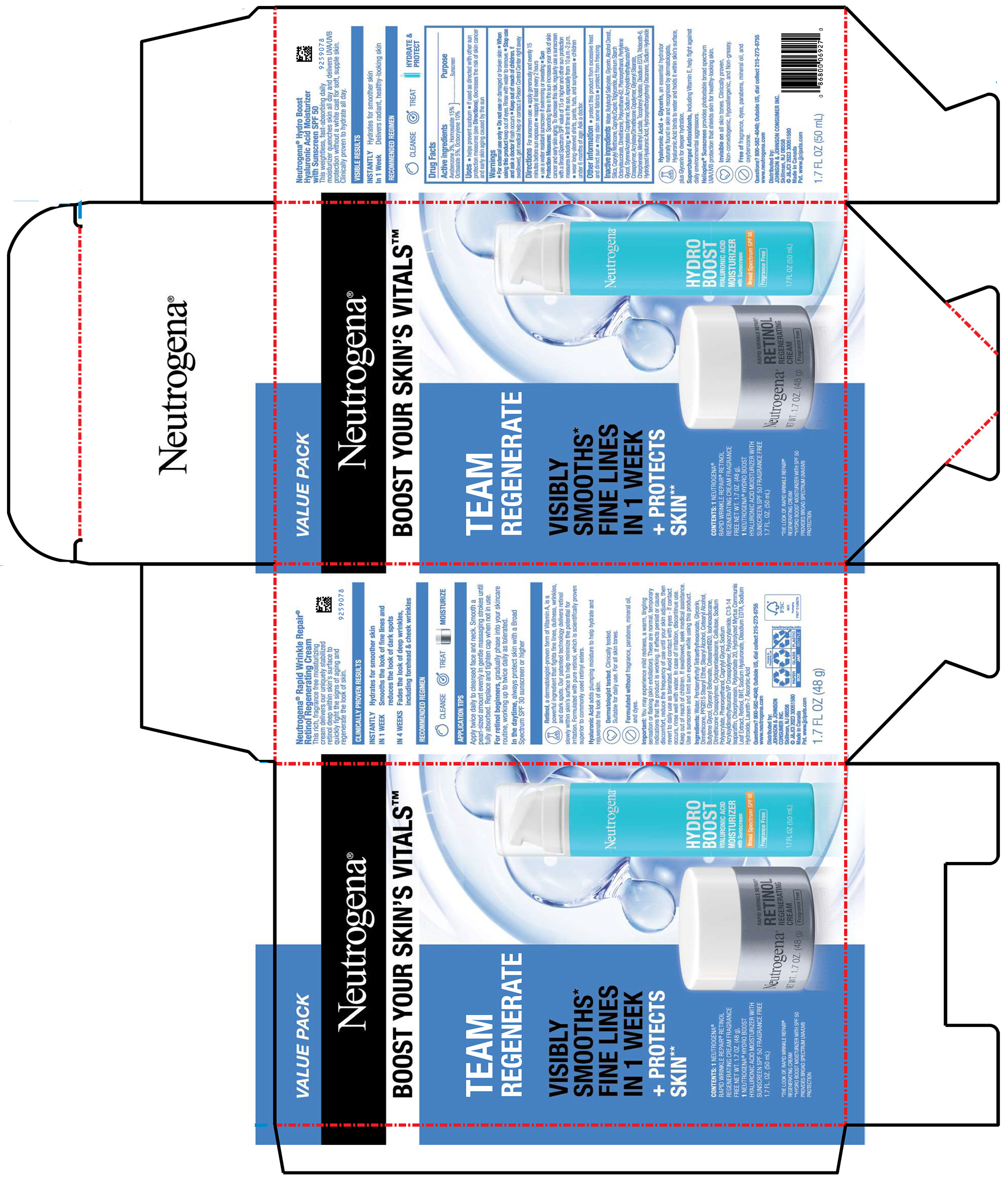
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - Kit Package
VALUE PACK
Neutrogena®
BOOST YOUR SKIN’S VITALS™
TEAM
REGENERATE
VISIBLY
SMOOTHS*
FINE LINES
IN 1 WEEK
+ PROTECTS
SKIN**
CONTENTS: 1 NEUTROGENA®
RAPID WRINKLE REPAIR® RETINOL
REGENERATING CREAM FRAGRANCE
FREE NET WT. 1.7 OZ. (48 g),
1 NEUTROGENA® HYDRO BOOST
HYALURONIC ACID MOISTURIZER WITH
SUNSCREEN SPF 50 FRAGRANCE FREE
1.7 FL. OZ. (50 mL)
*THE LOOK OF RAPID WRINKLE REPAIR®
REGENERATING CREAM
**HYDRO BOOST MOISTURIZER WITH SPF 50
PROVIDES BROAD SPECTRUM UVA/UVB
PROTECTION
INDICATIONS & USAGE SECTION
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
WARNINGS SECTION
Warnings
For external use only
Do not use on damaged or broken skin
When using this productkeep out of eyes. Rinse with water to remove.
Stop use and ask a doctor ifrash occurs
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
SPL UNCLASSIFIED SECTION
Distributed by:
JOHNSON & JOHNSON CONSUMER INC.
Skillman, NJ 08558
OTC - ACTIVE INGREDIENT SECTION
Active ingredients
Avobenzone 3%
Homosalate 15%
Octisalate 5%
Octocrylene 10%
OTC - PURPOSE SECTION
Purpose
Sunscreen
DOSAGE & ADMINISTRATION SECTION
Directions
For sunscreen use:
- apply generously and evenly 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
***Sun Protection Measures:**Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months of age: Ask a doctor.
STORAGE AND HANDLING SECTION
Other information
- protect this product from excessive heat and direct sun
- may stain some fabrics
- protect from freezing
INACTIVE INGREDIENT SECTION
Inactive ingredients
Water, Butyloctyl Salicylate, Glycerin, Alcohol Denat., Silica, Caprylyl Methicone, Caprylic/Capric Triglyceride, Aluminum Starch Octenylsuccinate, Dimethicone, Polyurethane-62, Phenoxyethanol, Pentylene Glycol, Styrene/Acrylates Copolymer, Sodium Acryloyldimethyltaurate/VP Crosspolymer, Acrylates/Dimethicone Copolymer, Glyceryl Stearate, Chlorphenesin, Menthyl Lactate, Tocopheryl Acetate, Disodium EDTA, Trideceth-6, Hydrolyzed Hyaluronic Acid, Hydroxymethoxyphenyl Decanone, Sodium Hydroxide
OTC - QUESTIONS SECTION
Questions?
Call toll-free800-582-4048 or 215-273-8755 (collect) or visit www.neutrogena.com
