Fludrocortisone Acetate
Fludrocortisone Acetate Tablets, USP Rx Only 8228801/0525F
001e72ea-2c9e-468c-90cc-6c347234e2a9
HUMAN PRESCRIPTION DRUG LABEL
Jan 20, 2023
American Health Packaging
DUNS: 929561009
Products 1
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Fludrocortisone Acetate
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (5)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel — Blister — 0.1 mg

Fludrocortisone
Acetate Tablet, USP
0.1 mg
SPL UNCLASSIFIED SECTION
PACKAGING INFORMATION
American Health Packaging unit dose blisters (see How Supplied section)
contain drug product from Amneal Pharmaceuticals LLC as follows:
(0.1 mg / 50 UD) NDC 68084-288-65 packaged from NDC 0115-7033
(0.1 mg / 100 UD) NDC 68084-288-01 packaged from NDC 0115-7033
Distributed by:
American Health Packaging
Columbus, OH 43217
8228801/0525F
DESCRIPTION SECTION
DESCRIPTION
Fludrocortisone acetate tablets USP, 0.1 mg contain fludrocortisone acetate, USP a synthetic adrenocortical steroid possessing very potent mineralocorticoid properties and high glucocorticoid activity; it is used only for its mineralocorticoid effects. The chemical name for fludrocortisone acetate is 9-fluoro-11β, 17, 21-trihydroxypregn-4-ene-3, 20-dione 21-acetate; its structural formula is:
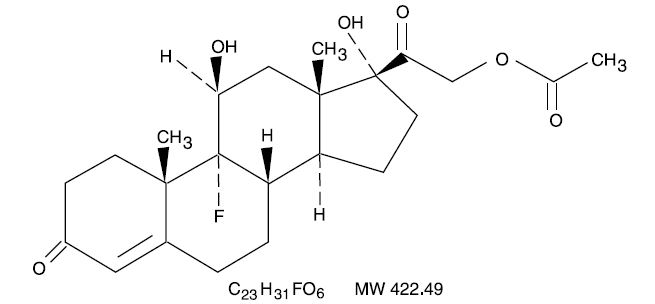
Fludrocortisone acetate tablets USP, 0.1 mg are available for oral administration as scored tablets providing 0.1 mg fludrocortisone acetate, USP per tablet. Inactive ingredients: croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF, and microcrystalline cellulose NF.
