Trazodone Hydrochloride
These highlights do not include all the information needed to use TRAZODONE HYDROCHLORIDE TABLETS safely and effectively. See full prescribing information for TRAZODONE HYDROCHLORIDE TABLETS. TRAZODONE HYDROCHLORIDE tablets, for oral use Initial U.S. Approval: 1981
094ca37b-3ae2-4889-9536-e7e5f20e3105
HUMAN PRESCRIPTION DRUG LABEL
Sep 7, 2023
American Health Packaging
DUNS: 929561009
Products 3
Detailed information about drug products covered under this FDA approval, including NDC codes, dosage forms, ingredients, and administration routes.
Trazodone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Trazodone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Trazodone Hydrochloride
Product Details
FDA regulatory identification and product classification information
FDA Identifiers
Product Classification
Product Specifications
INGREDIENTS (7)
Drug Labeling Information
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Package/Label Display Panel – Blister – 150 mg

traZODONE
Hydrochloride
Tablets, USP150 mg
WARNINGS AND PRECAUTIONS SECTION
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Pediatric and Young Adult Patients
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and over 4,400 pediatric patients, the incidence of suicidal thoughts and behaviors in pediatric and young adult patients was greater in antidepressant-treated patients than in placebo-treated patients. The drug- placebo differences in the number of cases of suicidal thoughts and behaviors per 1,000 patients treated are provided in Table 1.
No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about antidepressant drug effect on suicide.
Table 1 Risk Differences of the Number of Cases of Suicidal Thoughts or Behaviors in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients|
Age Range (years) |
Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1,000 Patients Treated |
|
Increases Compared to Placebo | |
|
< 18 |
14 additional patients |
|
18 to 24 |
5 additional patients |
|
Decreases Compared to Placebo | |
|
25 to 64 |
1 fewer patient |
|
≥ 65 |
6 fewer patients |
It is unknown whether the risk of suicidal thoughts and behaviors in pediatric and young adult patients extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression.
Monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing trazodone, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Serotonin Syndrome
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and SSRIs, including trazodone, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, and St. John's Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications (4), Drug Interactions (7.1)]. Serotonin syndrome can also occur when these drugs are used alone.
Serotonin syndrome signs and symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of trazodone with MAOIs is contraindicated. In addition, do not initiate trazodone in a patient being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking trazodone, discontinue trazodone before initiating treatment with the MAOI [see Contraindications (4), Drug Interactions (7.1)].
Monitor all patients taking trazodone for the emergence of serotonin syndrome. Discontinue treatment with trazodone and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of trazodone with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
5.3 Cardiac Arrhythmias
Clinical studies indicate that trazodone hydrochloride may be arrhythmogenic in patients with preexisting cardiac disease. Arrhythmias identified include isolated PVCs, ventricular couplets, tachycardia with syncope, and torsade de pointes. Postmarketing events, including torsade de pointes have been reported at doses of 100 mg or less with the immediate-release form of trazodone. Trazodone should also be avoided in patients with a history of cardiac arrhythmias, as well as other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death, including symptomatic bradycardia, hypokalemia or hypomagnesemia, and the presence of congenital prolongation of the QT interval. Trazodone is not recommended for use during the initial recovery phase of myocardial infarction. Caution should be used when administering trazodone to patients with cardiac disease and such patients should be closely monitored, since antidepressant drugs (including trazodone) may cause cardiac arrhythmias [see Adverse Reactions (6.2)].
Trazodone prolongs the QT/QTc interval. The use of trazodone should be avoided in patients with known QT prolongation or in combination with other drugs that are inhibitors of CYP3A4 (e.g., itraconazole, clarithromycin, voriconazole), or known to prolong QT interval including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), certain antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and certain antibiotics (e.g., gatifloxacin). Concomitant administration of drugs may increase the risk of cardiac arrhythmia [see Drug Interactions (7.1)].
5.4 Orthostatic Hypotension and Syncope
Hypotension, including orthostatic hypotension and syncope has been reported in patients receiving trazodone hydrochloride. Concomitant use with an antihypertensive may require a reduction in the dose of the antihypertensive drug.
5.5 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including trazodone, increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDS), other antiplatelet drugs, warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to drugs that interfere with serotonin reuptake have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the risk of bleeding associated with the concomitant use of trazodone and antiplatelet agents or anticoagulants. For patients taking warfarin, carefully monitor coagulation indices when initiating, titrating, or discontinuing trazodone.
5.6 Priapism
Cases of priapism (painful erections greater than 6 hours in duration) have been reported in men receiving trazodone. Priapism, if not treated promptly, can result in irreversible damage to the erectile tissue. Men who have an erection lasting greater than 4 hours, whether painful or not, should immediately discontinue the drug and seek emergency medical attention [see Adverse Reactions (6.2), Overdosage (10)].
Trazodone should be used with caution in men who have conditions that might predispose them to priapism (e.g., sickle cell anemia, multiple myeloma, or leukemia), or in men with anatomical deformation of the penis (e.g., angulation, cavernosal fibrosis, or Peyronie's disease).
5.7 Activation of Mania or Hypomania
In patients with bipolar disorder, treating a depressive episode with trazodone or another antidepressant may precipitate a mixed/manic episode. Activation of mania/hypomania has been reported in a small proportion of patients with major affective disorder who were treated with antidepressants. Prior to initiating treatment with trazodone, screen patients for any personal or family history of bipolar disorder, mania, or hypomania [see Dosage and Administration (2.3)] .
5.8 Discontinuation Syndrome
Adverse reactions after discontinuation of serotonergic antidepressants, particularly after abrupt discontinuation, include: nausea, sweating, dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), tremor, anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures. A gradual reduction in dosage rather than abrupt cessation is recommended whenever possible [See Dosage and Administration (2.6)].
5.9 Potential for Cognitive and Motor Impairment
Trazodone may cause somnolence or sedation and may impair the mental and/or physical ability required for the performance of potentially hazardous tasks. Patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that the drug treatment does not affect them adversely.
5.10 Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including trazodone may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Avoid use of antidepressants, including trazodone, in patients with untreated anatomically narrow angles.
5.11 Hyponatremia
Hyponatremia may occur as a result of treatment with SNRIs and SSRIs, including trazodone. Cases with serum sodium lower than 110 mmol/L have been reported. Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH).
In patients with symptomatic hyponatremia, discontinue trazodone and institute appropriate medical intervention. Elderly patients, patients taking diuretics, and those who are volume-depleted may be at greater risk of developing hyponatremia with SSRIs and SNRIs [see Use in Specific Populations (8.5)].
***Serotonin Syndrome:**Increased risk when co-administered with other serotonergic agents (e.g., SSRI, SNRI, triptans), but also when taken alone. If it occurs, discontinue trazodone and initiate supportive treatment (5.2). ***Cardiac Arrhythmias:**Increases the QT interval. Avoid use with drugs that also increase the QT interval and in patients with risk factors for prolonged QT interval (5.3) ***Orthostatic Hypotension and Syncope:**Warn patients of risk and symptoms of hypotension (5.4). ***Increased Risk of Bleeding:**Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), other antiplatelet drugs, warfarin, and other anticoagulants may increase this risk (5.5). ***Priapism:**Cases of painful and prolonged penile erections and priapism have been reported. Immediate medical attention should be sought if signs and symptoms of prolonged penile erections or priapism are observed (5.6). ***Activation of Mania or Hypomania:**Screen for bipolar disorder and monitor for mania or hypomania (5.7). ***Potential for Cognitive and Motor Impairment:**Has potential to impair judgment, thinking, and motor skills. Advise patients to use caution when operating machinery (5.9). ***Angle-Closure Glaucoma:**Avoid use of antidepressants, including trazodone, in patients with untreated anatomically narrow angles. (5.10).
DRUG ABUSE AND DEPENDENCE SECTION
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Trazodone hydrochloride tablets are not a controlled substance.
9.2 Abuse
Although trazodone hydrochloride has not been systematically studied in preclinical or clinical studies for its potential for abuse, no indication of drug-seeking behavior was seen in the clinical studies with trazodone hydrochloride.
CLINICAL PHARMACOLOGY SECTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
The mechanism of trazodone's antidepressant action is not fully understood, but is thought to be related to its enhancement of serotonergic activity in the CNS. Trazodone is both a selective serotonin reuptake inhibitor (SSRI) and a 5HT2 receptor antagonist and the net result of this action on serotonergic transmission and its role in trazodone's antidepressant effect is unknown.
12.2 Pharmacodynamics
Preclinical studies have shown that trazodone selectively inhibits neuronal reuptake of serotonin (Ki = 367 nM) and acts as an antagonist at 5-HT-2A (Ki = 35.6 nM) serotonin receptors. Trazodone is also an antagonist at several other monoaminergic receptors including 5-HT2B (Ki = 78.4 nM), 5-HT2C (Ki = 224 nM), α1A (Ki = 153 nM), α2C (Ki = 155 nM) receptors and it is a partial agonist at 5HT1A (Ki = 118 nM) receptor.
Trazodone antagonizes alpha 1-adrenergic receptors, a property which may be associated with postural hypotension.
12.3 Pharmacokinetics
Absorption
In humans, trazodone hydrochloride is absorbed after oral administration
without selective localization in any tissue. When trazodone hydrochloride is
taken shortly after ingestion of food, there may be an increase in the amount
of drug absorbed, a decrease in maximum concentration and a lengthening in the
time to maximum concentration. Peak plasma levels occur approximately one hour
after dosing when trazodone hydrochloride is taken on an empty stomach or 2
hours after dosing when taken with food.
Metabolism
In vitro studies in human liver microsomes show that trazodone is metabolized,
via oxidative cleavage, to an active metabolite, m-chlorophenylpiperazine
(mCPP) by CYP3A4. Other metabolic pathways that may be involved in the
metabolism of trazodone have not been well characterized. Trazodone is
extensively metabolized; less than 1% of an oral dose is excreted unchanged in
the urine.
Elimination
In some patients trazodone may accumulate in the plasma.
Protein Binding
Trazodone is 89 to 95% protein bound in vitro at concentrations attained with
therapeutic doses in humans.
NONCLINICAL TOXICOLOGY SECTION
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No drug- or dose-related occurrence of carcinogenesis was evident in rats
receiving trazodone in daily oral doses up to 7.3 times the maximum
recommended human dose (MRHD) of 400 mg/day in adults on a mg/m 2 basis.
Mutagenesis
No genotoxicity studies were conducted with trazodone.
Impairment of Fertility
Trazodone has no effect on fertility in rats at doses up to 7.3 times the MRHD
in adults on a mg/m 2 basis.
HOW SUPPLIED SECTION
16 HOW SUPPLIED/STORAGE AND HANDLING
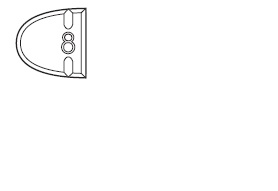
Trazodone Hydrochloride Tablets USP, 50 mg are white to off-white, round-
shape, biconvex beveled tablets, bisect on one side and plain on other side.
The bisected side of tablet is debossed with '8' on upper side of bisect and
'05' on lower side of bisect and are supplied as follows:
Unit dose packages of 100 (10 x 10) NDC 60687-443-01
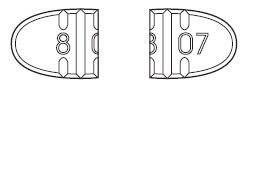
Trazodone Hydrochloride Tablets USP, 100 mg are white to off-white, round-
shape, biconvex beveled tablets, bisect on one side and plain on other side.
The bisected side of tablet is debossed with '8' on upper side of bisect and
'06' on lower side of bisect and are supplied as follows:
Unit dose packages of 100 (10 x 10) NDC 60687-454-01
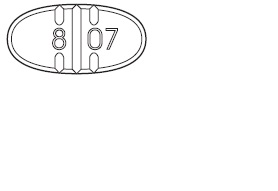
Trazodone Hydrochloride Tablets USP, 150 mg are white to off-white, oval-
shape, flat faced beveled tablets having one full bisect and two trisect
notches on one side and two trisects on other side. The full bisected side of
tablet is debossed with '8' on one side of bisect and '07' on other bisect
segments and are supplied as follows:
Unit dose packages of 100 (10 x 10) NDC 60687-432-01
Directions for using the correct score when breaking the tablet, please refer to the following:
-For 50 mg, break the score on either the left or right side of the tablet (one-third of a tablet).
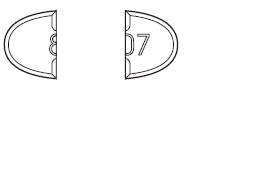
-For 75 mg, break the score down the middle of the tablet (one-half of a tablet).
-For 100 mg, break the score on either the left or right side of the tablet (two-thirds of a
tablet).
-For 150 mg, use the entire tablet.
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature].
**FOR YOUR PROTECTION:**Do not use if blister is torn or broken.
INFORMATION FOR PATIENTS SECTION
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Suicidal Thoughts and Behaviors
Advise patients and caregivers to look for the emergence of suicidality,
especially early during treatment and when the dosage is adjusted up or down
and instruct them to report such symptoms to the healthcare provider [see Box Warning and Warnings and Precautions (5.1)].
Dosage and Administration
Advise patients that trazodone hydrochloride tablets should be taken shortly
after a meal or light snack. Advise patients regarding the importance of
following dosage titration instructions [see Dosage and Administration (2)].
Serotonin Syndrome
Caution patients about the risk of serotonin syndrome, particularly with the
concomitant use of trazodone hydrochloride tablets with other serotonergic
drugs including triptans, tricyclic antidepressants, fentanyl, lithium,
tramadol, tryptophan, buspirone, St. John's Wort, and with drugs that impair
metabolism of serotonin (in particular, MAOIs, both those intended to treat
psychiatric disorders and also others, such as linezolid). Patients should
contact their health care provider or report to the emergency room if they
experience signs or symptoms of serotonin syndrome [see Warnings and Precautions (5.2) and Drug Interactions (7)].
Activation of Mania/Hypomania
Advise patients and their caregivers to observe for signs of activation of
mania/hypomania and instruct them to report such symptoms to the healthcare
provider [see Warnings and Precautions (5.7)].
Increased Risk of Bleeding
Inform patients about the concomitant use of trazodone hydrochloride tablets
with aspirin, NSAIDs, other antiplatelet drugs, warfarin, or other
anticoagulants because the combined use of drugs that interfere with serotonin
reuptake and these medications has been associated with an increased risk of
bleeding. Advise them to inform their health care providers if they are taking
or planning to take any prescription or over-the-counter medications that
increase the risk of bleeding [see Warnings and Precautions (5.5)].
Discontinuation Syndrome
Advise patients not to abruptly discontinue trazodone hydrochloride tablets
and to discuss any tapering regimen with their healthcare provider. Adverse
reactions can occur when trazodone hydrochloride tablets are discontinued [see Warnings and Precautions (5.8)].
Concomitant Medications
Advise patients to inform their health care providers if they are taking, or
plan to take any prescription or over-the-counter medications since there is a
potential for interactions [see Drug Interactions (7.1)].
Pregnancy
Advise patients to notify their healthcare provider if they become pregnant or
intend to become pregnant during therapy with trazodone hydrochloride tablets.
Advise patients that there is a pregnancy exposure registry that monitors
pregnancy outcomes in women exposed to trazodone hydrochloride tablets during
pregnancy [see Use in Special Populations (8.1)].
